9.1 CHROMOSOMES: AN OVERVIEW
Chromosomes are typically the largest macromolecules in any cell, by a considerable margin. Why must chromosomes be so large? They contain the blueprints for an organism, and thus a great deal of information is contained within them. But the genes in each chromosome constitute only part of that information. The chromosomes themselves are macromolecular entities that must be synthesized, packaged, protected, and properly distributed to daughter cells at cell division. Significant segments of every chromosome are dedicated to these functions. All aspects of chromosome function are affected by the reality of chromosome size.
Chromosome Function Relies on Specialized Genomic Sequences
The chromosomes of cells and viruses come in several forms. Bacterial chromosomes are often circular (in the sense of an endless loop rather than a perfect circle). Most eukaryotic chromosomes are linear. In viruses we find additional variations, including both single-
Genes provide the information to specify all the RNAs and proteins produced in a given cell, but other DNA sequences in the genome are dedicated to the maintenance of the chromosome itself: initiation and termination of replication, segregation during cell division, and, where necessary, protection and maintenance of the chromosome ends. In bacteria, an origin of replication provides a start site for chromosomal replication (see Figure 8-1). Specialized replication-
299
The centromere is a segment of each eukaryotic chromosome that functions during cell division as an attachment point for proteins that link the chromosome to the mitotic spindle at metaphase (Figure 9-1). This attachment is essential for the equal and orderly distribution of chromosome sets to daughter cells. (See Chapter 2 for a review of the events of mitosis.) The centromeres of Saccharomyces cerevisiae have been isolated and studied. The sequences essential to centromere function are about 130 bp long and very rich in A = T base pairs. The centromere sequences of higher eukaryotes are much longer and, unlike those of yeast, generally contain regions of simple-
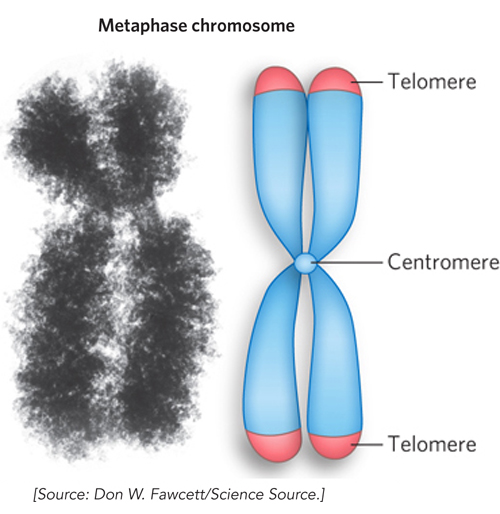
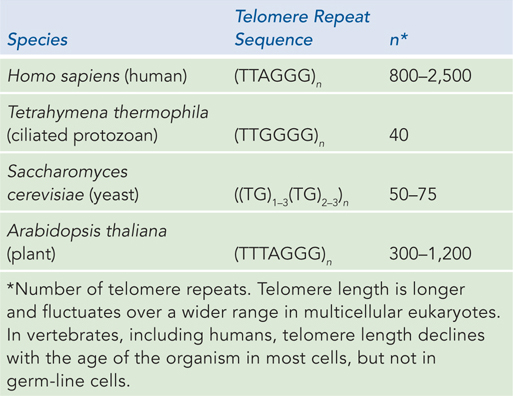
Telomeres are sequences at the ends of eukaryotic chromosomes that add stability by protecting the ends from nucleases and providing unique mechanisms for the faithful replication of linear DNA molecules. DNA polymerases cannot synthesize DNA to the very ends of a linear chromosome (see Chapter 11). Solving the end-
5′-(TxGy)n
3′-(AxCy)n
where x and y are generally between 1 and 4 (Table 9-1) and the number of telomere repeats, n, is in the range of 20 to 100 for most single-
Artificial chromosomes provide a means of better understanding the functional significance of many structural features of eukaryotic chromosomes. A reasonably stable artificial linear chromosome requires only three components: a centromere, a telomere at each end, and an appropriate number of replication origins. Yeast artificial chromosomes (YACs) have been developed as a research tool in biotechnology (see Figure 7-7). YACs have also been useful in confirming the critical functions of centromeres and telomeres. Building on this foundation, researchers have constructed human artificial chromosomes (HACs). HACs are reasonably stable when introduced into a human tissue culture cell line, if they include human centromere and telomere sequences in addition to active replication origins.
Continued development of HACs, particularly of their efficient introduction into human cells, may eventually provide new avenues for the treatment of genetic diseases. Most genetic diseases can be traced to an alteration in one or more particular genes that changes or eliminates their function. Efforts to directly remove such genes in human cells and replace them with normal, functional versions at the correct chromosomal locus have met with limited success. Nonetheless, there are ongoing efforts to improve this process of correcting disease-
300
Chromosomes Are Longer Than the Cellular or Viral Packages Containing Them
The observation that genomic DNAs are orders of magnitude longer than the cells or viruses that contain them applies to every class of organism and viral parasite. Lengths of double-
Given the magnitude of the one-
KEY CONVENTION
Molecular biology involves structures with dimensions that are small fractions of a meter. One-
Viruses Viruses are not free-
Almost all plant viruses and some bacterial and animal viruses have RNA genomes, and they are quite small. For example, the genomes of mammalian retroviruses, such as HIV, have about 9,000 nucleotides, and the genome of the bacteriophage Qβ has 4,220 nucleotides. However, even these small nucleic acids have total lengths of about 3 and 1.4 μm, respectively. In comparison, the protein coat of HIV is about 100 nm in diameter, and that of Qβ is about 26 nm, so the RNAs are 30 to 50 times longer than their viral protein coats. Both types of virus have linear, single-
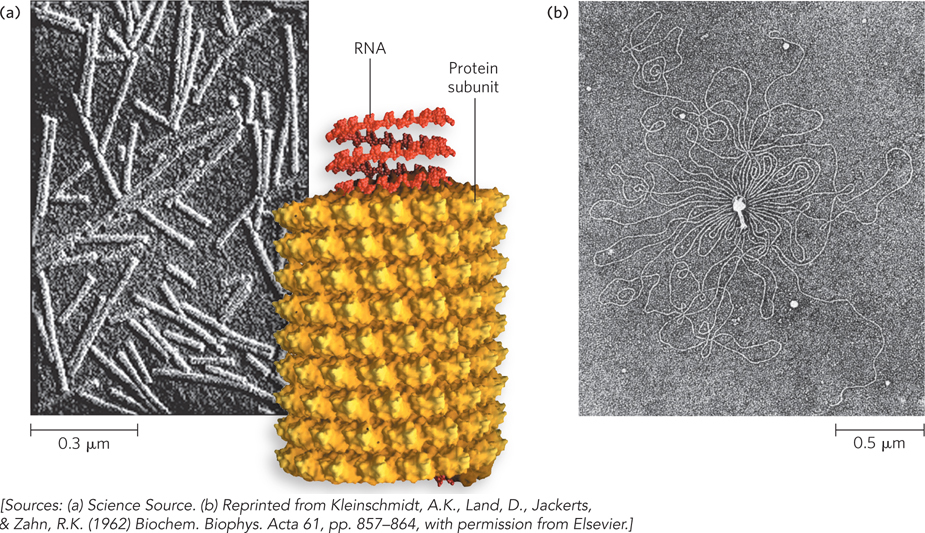
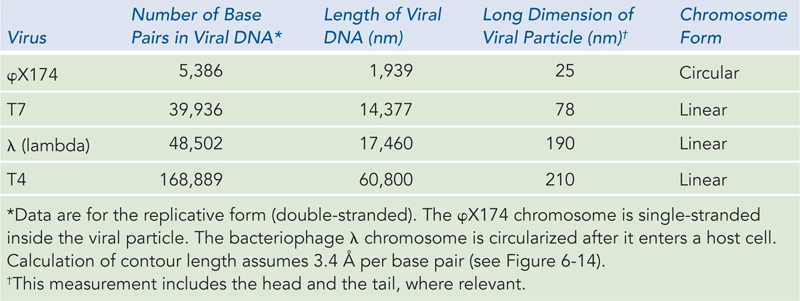
The genomes of DNA viruses vary greatly in size and form (see Table 8-1), but all are longer than the viral capsid heads that enclose them. Many viral DNAs are circular for at least part of their life cycle. During viral replication inside a host cell, specific types of viral DNA, called replicative forms, may appear; for example, many linear DNAs become circular, and all single-
301
Bacteria A single E. coli cell contains almost 100 times more DNA than a bacteriophage λ particle (see Table 9-2). The chromosome of the most common E. coli strain studied in laboratories worldwide (K-
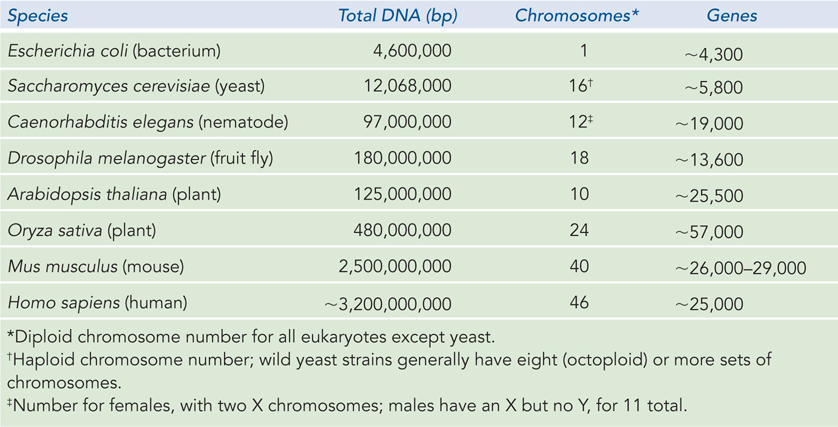
302
Eukaryotes A yeast cell, one of the simplest eukaryotes, has 2.6 times more DNA in its genome than an E. coli cell (see Table 9-3). Cells of Drosophila melanogaster, the fruit fly used in classical genetics studies, contain more than 35 times as much DNA as E. coli cells, and human cells have almost 700 times as much. The cells of many plants and amphibians contain even more. All of this DNA must fit into a eukaryotic cell that is typically 10 to 20 μm across (although size can vary greatly, even within a single organism). The genetic material of eukaryotic cells is apportioned into multiple chromosomes, the diploid (2n) number depending on the species. A human somatic cell, for example, has 46 chromosomes (Figure 9-4). Each chromosome of a eukaryotic cell contains a single, very large, duplex DNA molecule. The DNA molecules in the 24 different types of human chromosomes (22 matching pairs plus the X and Y sex chromosomes) vary in length over a 25-

303
HIGHLIGHT 9-1 MEDICINE: The Dark Side of Antibiotics
Over the course of the twentieth century, the average life expectancy for people in the developed countries increased by 10 years, and the development of antibiotics for the treatment of infectious diseases was a major contributor to this improved longevity. Ironically, an overuse of antibiotics is now leading to their demise as useful therapeutics, as bacterial pathogens evolve to develop antibiotic resistance.
The most common vehicles for transmitting antibiotic-
Plasmids contain a range of sequences involved in their own propagation. These sequences often function in several related bacterial species, and the host range can increase with the aid of small numbers of mutations. When genes conferring antibiotic resistance are integrated into a plasmid, the plasmid becomes a vehicle for transferring the resistance element from one bacterium to another, and even between species. For example, plasmids carrying the gene for the enzyme β-lactamase confer resistance to β-lactam antibiotics, such as penicillin, ampicillin, and amoxicillin. Transfer of plasmids to other bacteria can occur through horizontal gene transfer, in which plasmids pass from an antibiotic-
Under the strong selective pressure brought about by widespread antibiotic treatments, bacterial pathogens can acquire antibiotic resistance rapidly. The extensive use of antibiotics in some human populations has encouraged the spread of antibiotic resistance–
Eukaryotic cells also have organelles that contain DNA. Mitochondria and chloroplasts carry their own genomic DNAs (Figure 9-5). The evolutionary origin of mitochondrial and chloroplast DNAs has been the subject of much speculation. A widely accepted hypothesis, proposed by Lynn Margulis, is that they are vestiges of the chromosomes of ancient bacteria that gained access to the cytoplasm of host cells and became the precursors of these organelles (see Figure 8-17a). Mitochondrial DNA (mtDNA) codes for mitochondrial tRNAs and rRNAs and a few mitochondrial proteins; more than 95% of mitochondrial proteins are encoded by nuclear DNA. Mitochondria and chloroplasts divide when the cell divides. Their DNA is replicated before and during cell division, and the daughter DNA molecules pass into the daughter organelles.
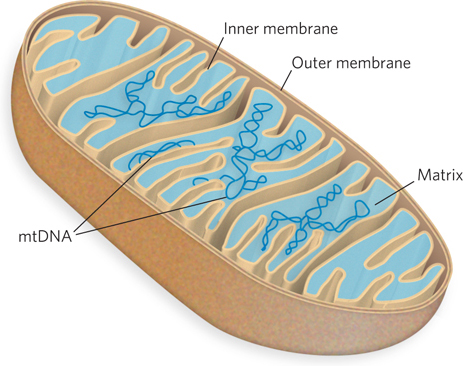

Mitochondrial DNA molecules are much smaller than nuclear chromosomes. In animal cells, mtDNA contains fewer than 20 kbp (16,569 bp in human mtDNA) and is a circular duplex. Each mitochondrion typically has 2 to 10 copies of the mtDNA, but the number can be much higher: hundreds in muscle cells, and 100,000 in a mature oocyte. In a few organisms (e.g., trypanosomes, the parasites that cause sleeping sickness), the mitochondrial DNA is particularly abundant and organized. These mitochondria contain thousands of copies of mtDNA organized into a complex interlinked matrix known as a kinetoplast. Plant cell mtDNA is much larger than that in animal cells, ranging from 200 to 2,500 kbp. Chloroplast DNA (cpDNA) exists as circular duplexes of 120 to 160 kbp. Organelle DNA, like nuclear DNA, undergoes considerable compaction: DNA molecules 5 to 500 μm long must be accommodated in organelles about 1 to 5 μm in diameter.
304
Some eukaryotes also contain plasmids; they have been found in yeast and some other fungi.
SECTION 9.1 SUMMARY
All cellular chromosomes contain sequences required for chromosome function, including replication origins. Bacterial chromosomes also contain termination sequences and other sequences necessary for chromosomal segregation during mitosis.
Eukaryotic chromosomes contain centromeres, which are attachment points for the mitotic spindle, and telomeres, specialized sequences at the ends of a chromosome that protect and stabilize the entire chromosome.
All genomic DNA and RNA molecules are longer—
often orders of magnitude longer— than the viral coats, organelles, and cells in which they are packaged. Viral genomes vary in nucleic acid (DNA or RNA), structure (single-
stranded or double- stranded), and length. Bacterial cells contain both genomic DNA (usually circular) and plasmids; both types are compacted in the cell and are replicated and segregated into daughter cells at cell division.
Eukaryotic chromosomes are linear and vary in number, depending on the species. Humans have 46 chromosomes, varying in length and condensed to fit into the cell nucleus. Mitochondria and chloroplasts contain their own circular genomes, in numbers ranging from several copies to hundreds of thousands of copies per organelle, depending on cell type.