18.1 THE RIBOSOME
In 1974, George Palade received a Nobel Prize for his discovery, in the 1950s, of ribosomes, the macromolecular machines responsible for protein synthesis (Figure 18-1). Although historically considered organelles, ribosomes are not encapsulated in a lipid membrane and thus are more accurately described as very large (macro) molecules. Ribosomes are present in the cytosol of all cells, as well as in the matrix of mitochondria and the stroma of chloroplasts. Because protein synthesis is common to virtually all life forms, ribosomes are evolutionarily well-conserved. In bacteria, they can translate mRNA as it is being transcribed from DNA, but in eukaryotes, the nuclear envelope and mRNA processing steps separate RNA synthesis from translation (RNA processing is discussed in Chapter 16). Ribosomes are abundant; each E. coli cell contains approximately 15,000 ribosomes, which make up almost 25% of the dry weight of the cell. The large number of these macromolecules is essential to the cell’s ability to produce proteins when needed. The structure of the ribosome facilitates protein synthesis by bringing together the mRNA codon and the corresponding charged tRNA adaptor, and then catalyzing peptide bond formation. Although the general function of ribosomes has been well-established for some time, advances in understanding the structure and assembly of the ribosome have elucidated more of the details of protein synthesis. Here we provide an overview of ribosomal structure and function that will serve as a framework for the details in the rest of the chapter.
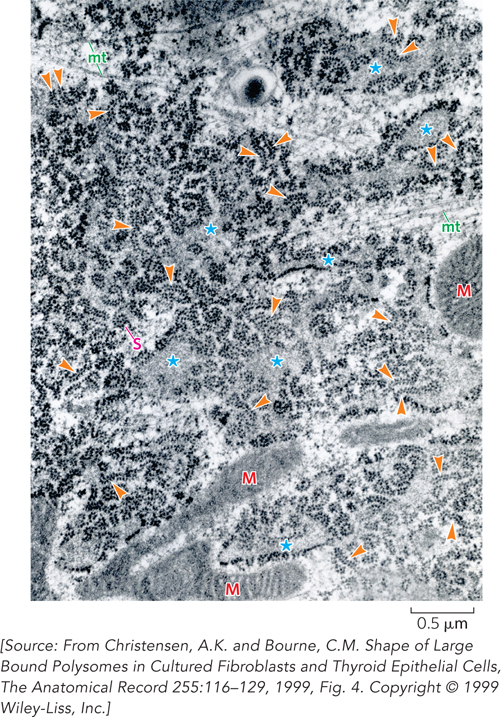
Figure 18-1: Ribosomes. Many of the ribosomes in a cell’s cytoplasm are assembled on the endoplasmic reticulum, the cell’s “shipping and handling” center for membrane protein production. In this electron micrograph, arrows indicate large polyribosomes, or polysomes, which consist of many ribosomes lined up on a single messenger RNA. Mitochondria (M) and microtubules (mt) are also visible, as well as large spiral polyribosomes (S). The interior (lumen) of the rough endoplasmic reticulum often appears expanded (asterisks).
The Ribosome Is an RNA-Protein Complex Composed of Two Subunits
Bacterial ribosomes contain about 60% ribosomal RNA and 40% protein, organized into two unequal subunits that are named according to their sedimentation coefficients (in svedberg units, S; see Chapter 16). The 50S subunit, the larger of the two, contains the peptidyl transferase center, which catalyzes peptide bond formation between adjacent amino acids in a growing polypeptide chain. The 30S subunit contains the decoding center where aminoacylated tRNAs “read” the genetic code by base pairing with each triplet codon in the mRNA. The assembled ribosome, with a combined sedimentation coefficient of 70S, smoothly integrates the functions of each subunit to ensure rapid, accurate protein synthesis.
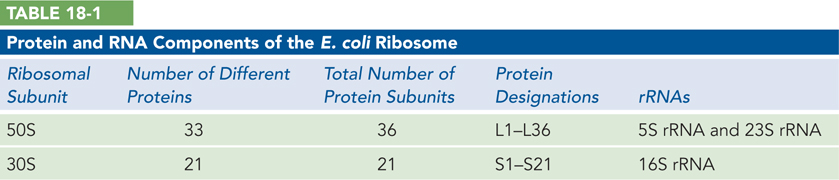
Each ribosomal subunit contains dozens of ribosomal proteins (r-proteins) and one or more large ribosomal RNAs (rRNAs) (Table 18-1). Like the subunits themselves, rRNAs are named in svedberg units. The 50S subunit contains a 5S and a 23S rRNA, and the 30S subunit contains a single 16S rRNA; these RNAs together comprise more than 4,500 nucleotides. The 50S subunit in E. coli also contains 36 different r-proteins, named L1 through L36 (L for large subunit), and the 30S subunit contains a single copy of each of 21 different r-proteins, named S1 through S21 (S for small subunit). Although there are many more individual r-proteins than RNAs in the ribosome, the relatively small size of most r-proteins (∼15 kDa, on average, in bacteria) means that the proteins contribute only about one-third of the overall mass of the ribosome.
Decades of research on the structure and function of ribosomal proteins and RNAs have shifted the focus from the proteins to the rRNA. Despite the complexity of ribosome structure, Masayasu Nomura and colleagues demonstrated in the late 1960s that both bacterial ribosomal subunits can be broken down into their RNA and protein components, then reconstituted in vitro. Under appropriate experimental conditions, the RNAs and proteins spontaneously reassemble to form 50S or 30S subunits nearly identical in sedimentation behavior and activity to native subunits. Sequencing of rRNAs in the 1970s, and the secondary structure proposed for 16S rRNA by Harry Noller and Carl Woese in 1981, showed that rRNAs have been highly conserved by evolution, particularly in molecular regions implicated in the critical functions of the ribosome (Figure 18-2). Noller’s biochemical analysis of functional inactivation of ribosomes provided the first evidence for the fundamental role of rRNA in catalyzing protein synthesis (see this chapter’s Moment of Discovery). Along with Woese’s sequence analysis of rRNAs from a range of organisms, these data also provided an important tool for analyzing evolutionary relationships among species.
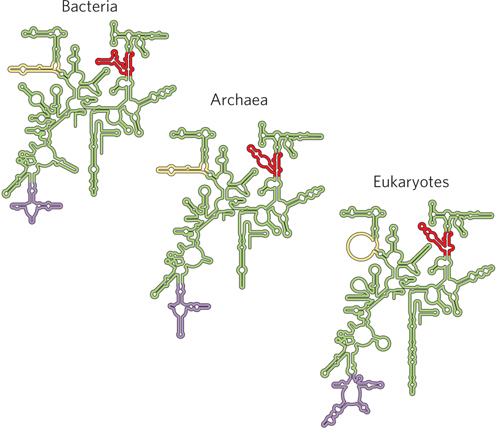
Figure 18-2: Conservation in the secondary structure of small-subunit rRNAs from the three domains of life. Red, yellow, and purple indicate areas where the structures of bacterial, archaeal, and eukaryotic rRNAs have diverged; conserved regions are shown in green.
In the culmination of many years of work in multiple laboratories, cryo-electron microscopy (using frozen samples) and x-ray crystallography have now revealed the atomic-resolution structure of the bacterial ribosome and its subunits in exquisite detail (Figure 18-3a). Venki Ramakrishnan, Tom Steitz, and Ada Yonath received a Nobel Prize in 2009 for their crystallographic work. More recently, the crystal structure of a eukaryotic ribosome was solved, revealing a similar overall structure but with greater complexity that reflects additional levels of regulation (Figure 18-3b).
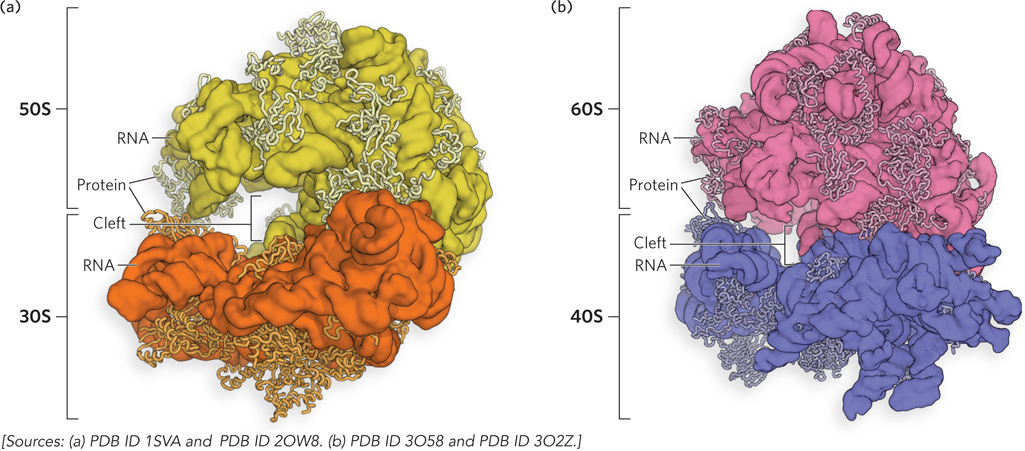
Figure 18-3: Crystal structures of bacterial and eukaryotic ribosomes. (a) In bacteria, the 50S (large) and 30S (small) subunits together form the 70S ribosome. The interface between the two subunits forms a cleft where the peptidyl transferase reaction (described later in this chapter) occurs. (b) The yeast ribosome has a similar structure, with increased complexity.
With a combined molecular weight of ∼2.5 × 106 Da, the bacterial ribosome is far more complex than the DNA or RNA polymerases, and knowledge of its structure has provided a wealth of insights about its function and evolutionary origins. The two irregularly shaped ribosomal subunits fit together to form a cleft through which the mRNA passes during translation. Although the proteins in bacterial ribosomes vary in size and structure, most have globular domains arranged on the ribosomal surface. Some proteins also have snakelike extensions that protrude into the rRNA core of the ribosome, stabilizing its structure. The functions of some of these proteins have not yet been determined, but a structural role seems likely for many of them.
Each of the three single-stranded rRNAs of E. coli has a specific three-dimensional conformation with extensive intrachain base pairing. This secondary structure was predicted based on the available sequences of rRNAs from many organisms (see Figure 18-2). These structures have largely been confirmed in high-resolution three-dimensional models, yet they fail to convey the extensive network of tertiary interactions evident in the complete structure.
Extensive structural, biochemical, and genetic data support the conclusion that rRNA is responsible for all ribosomal functions, including tRNA binding and peptide bond formation. The predominant location of r-proteins on the outer surface of the ribosome, away from its RNA-rich functional interface, underscores this idea (see Figure 18-3). In addition to dominating the functional centers within each ribosomal subunit, rRNA provides most of the contacts between the two subunits in the intact ribosome. The intersubunit bridges not only hold the two subunits together but also foster relative movement between the subunits that is integral to the process of polypeptide elongation (see Section 18.4).
The ribosomes of eukaryotic cells (other than the mitochondrial and chloroplast ribosomes) are larger and more complex than bacterial ribosomes. Electron microscopic and centrifugation studies show that these ribosomes have a diameter of about 23 nm and a sedimentation coefficient of about 80S. Like their bacterial counterparts, eukaryotic ribosomes have two subunits. Subunit size varies among species, but, on average, the subunits are 60S and 40S. Altogether, the cytosolic ribosomes contain more than 80 different proteins and four types of rRNA; unlike bacterial ribosomes, they cannot be spontaneously reconstituted in vitro, suggesting a more complex assembly process. In contrast, the ribosomes of mitochondria and chloroplasts are somewhat smaller and simpler than bacterial ribosomes. Nevertheless, the rRNAs of all cell types are conserved (and, as we saw in Chapter 17, all tRNAs have similar sizes and shapes), indicating that ribosomal structure and function are strikingly similar in all organisms and organelles.
Ribosomal Subunits Associate and Dissociate in Each Cycle of Translation
The association of ribosomal subunits at the start of protein synthesis and their dissociation on release of the completed polypeptide are fundamental to the process of translation in all cells. Because the subunits are initially separated, the initiation of protein synthesis is inherently regulated by the assembly of active ribosomes on an mRNA, together with tRNAs. The recruitment of the small ribosomal subunit to an mRNA is an essential first step, and cells and viruses have a variety of mechanisms for controlling how and when this happens.
An overview of translation is shown in Figure 18-4. In general, translation—the production of a protein product—begins with mRNA and an initiator tRNA binding to the small ribosomal subunit. Once the small subunit is positioned at the beginning of the coding sequence of the mRNA, the large ribosomal subunit joins noncovalently to form an active ribosome that begins reading each mRNA codon in sequence in the 5′→3′ direction. As each codon is encountered, a matching tRNA bearing its covalently attached amino acid enters the decoding and peptidyl transferase centers of the ribosome. After peptide bond formation between the C-terminal end of the growing polypeptide chain and the amino acid of the incoming aminoacyl-tRNA, the ribosome translocates to the next codon, and the cycle repeats. On encountering a stop codon, the ribosome is released along with the completed protein product, the ribosomal subunits dissociate, and the process is ready to begin again on another mRNA.
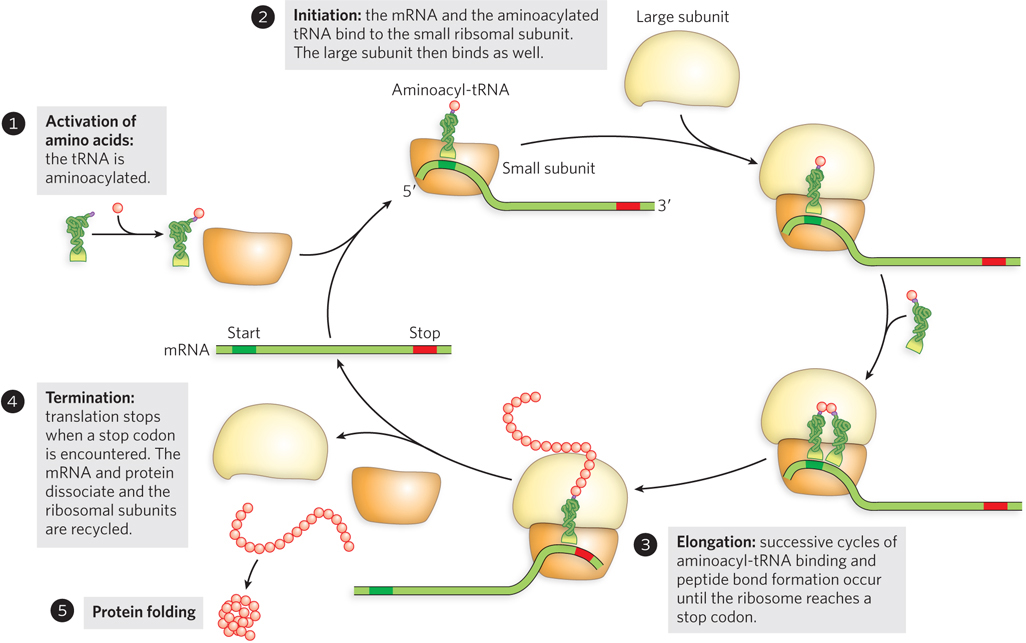
Figure 18-4: An overview of the main events in translation. Following the activation of amino acids by acylation to tRNAs (step 1), translation initiates with the assembly of mRNA and aminoacylated tRNA on the small ribosomal subunit, followed by joining with the large subunit to form an active ribosome (step 2). Polypeptide elongation occurs in successive cycles of aminoacyl-tRNA binding and peptide bond formation in the order directed by the genetic code in the mRNA (step 3). Translation stops when the ribosome encounters a stop codon in the mRNA, leading to release of the mRNA and dissociation of the ribosome into its two subunits (step 4); the polypeptide folds during or immediately following translation to form a functional protein (step 5).
Because mRNAs are usually at least 300 nucleotides long and extend well beyond the ∼200 Å girth of the ribosome, multiple ribosomes can occupy each mRNA during translation, forming a polysome, or polyribosome (Figure 18-5a). Polysomes can be visualized by electron microscopy, and because they form very high molecular weight particles, they are also readily detected in cell extracts by analyzing their sedimentation in sucrose or glycerol density gradients (Figure 18-5b). Polysome formation lets each mRNA molecule provide the template for multiple copies of a protein molecule at once, thus allowing the efficient use of each mRNA.
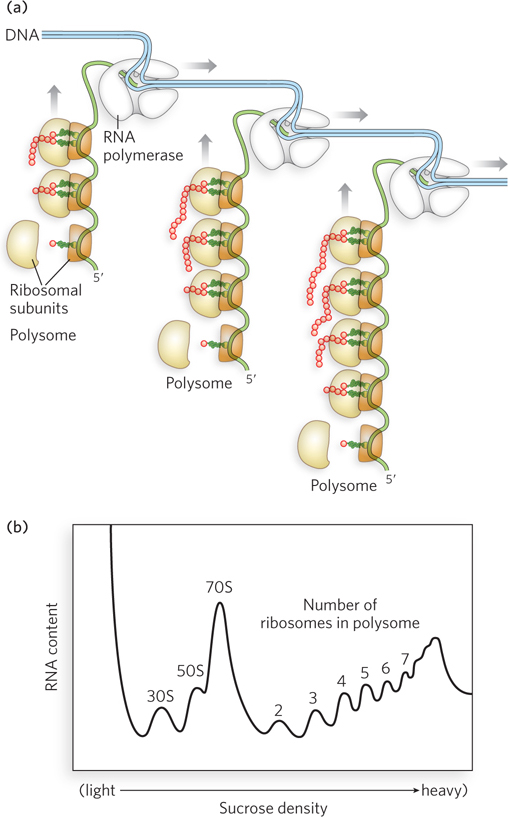
Figure 18-5: Polysomes. (a) Polysomes consist of multiple ribosomes associated with a single mRNA. (b) Polysomes can be separated on a sucrose density gradient. The polysome profile indicates total RNA content at increasing sucrose density. The results show that 30S and 50S ribosomal subunits and 70S ribosomes migrate near the top of the gradient, whereas polysomes, comprising two or more ribosomes associated with mRNA transcripts, migrate in the heavier (more dense) fractions of the gradient.
The Ribosome Is a Ribozyme
Noller concluded that ribosomal RNA has a fundamental importance in catalyzing protein synthesis, based on the results of two experiments. First, he inactivated the ribosome by making base changes in rRNA; second, he removed proteins from the ribosome and found that the deproteinized ribosome retained peptidyl transferase activity (see the How We Know section at the end of this chapter).
The object of the second experiment was to remove proteins without disrupting rRNA structure, which precluded the use of extraction reagents such as acid. Instead, detergents, a protease, or phenol were used, all of which are effective deproteinizing treatments that do not degrade or denature RNA. To monitor the activity of ribosomes after protein removal, Noller and his colleagues used a simplified peptidyl transferase reaction, referred to as the fragment reaction. This requires only the 50S subunit and does not need initiation or elongation factor proteins (factors discussed later in this chapter); the assay monitors the addition of [35S]methionine to an amino acid mimic, puromycin. Using the fragment reaction, the researchers found that the 50S subunit of Thermus aquaticus, a bacterium that grows at elevated temperatures (a thermophile), retained peptidyl transferase activity even after proteins were extracted by treatments with detergent, proteinase K (a nonspecific protease that degrades virtually all proteins), or phenol (Figure 18-6). The thermophilic ribosomes were selected because they were expected to be inherently more stable than ribosomes from organisms such as E. coli that grow at moderate temperatures. However, even E. coli 50S subunits remained active after treatment with detergent or proteinase K, suggesting that the rRNA structure was largely intact.
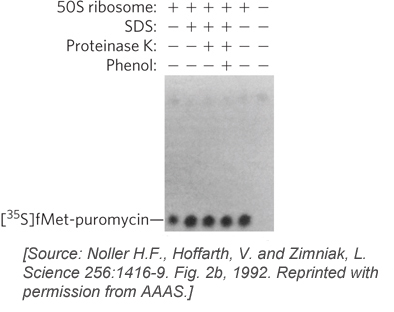
Figure 18-6: The effects of method of protein extraction on ribosomal function in vitro. Ribosomes from the bacterium Thermus aquaticus were treated with the detergent sodium dodecyl sulfate (SDS), the enzyme proteinase K, or phenol, then tested for peptidyl transferase activity using the fragment reaction. The product of the reaction, [35S]fMet-puromycin, was assayed by paper electrophoresis and autoradiography. (fMet is N-formylmethionine, the initiating amino acid in bacterial protein synthesis, described later in this chapter.)
These important findings foreshadowed the discovery that there is no protein within 18 Å of the peptidyl transferase active site in the crystal structure of the 50S ribosomal subunit (Figure 18-7). The high-resolution structure thus confirmed what had been suspected for more than a decade: the ribosome is a ribozyme. The ribosomal RNA, not protein, is responsible for catalysis of peptide bond formation.
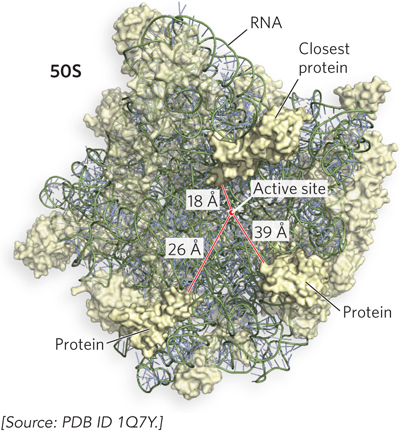
Figure 18-7: Ribosomal RNA in the ribosomal active site. The active site—where the peptidyl transferase reaction forms peptide bonds—is 18 Å away from the closest r-protein, evidence that the ribosome is a ribozyme.
In addition to confirming the central role of rRNA in peptide bond formation, crystal structures of the ribosome and its subunits show that most of the contacts between tRNA and the ribosome involve contacts with 16S or 23S rRNA, not with protein. Thus, with these new data, the traditional focus on the protein components of ribosomes shifted. In addition to providing the structural core, rRNAs form the functional core of the ribosome. This implies that r-proteins are secondary elements in the complex, playing a stabilizing or regulatory role rather than a catalytic role in the business of translation.
What do these findings suggest about the origin of this most fundamental cellular process? Francis Crick wondered in the 1960s about the possible existence of an all-RNA ribosome at some early point in evolution. The importance of rRNA in modern ribosomes supports this idea and is consistent with the addition of proteins to a preexisting rRNA-containing ribosome over the course of evolution. Recent studies of mitochondrial ribosomes suggest that r-proteins may restrict the variety of proteins that a ribosome can synthesize (Highlight 18-1).
HIGHLIGHT 18-1 EVOLUTION: Mitochondrial Ribosomes: A Window into Ribosome Evolution?

Rajendra Agrawal

Stephen Harvey
Mitochondria encode their own ribosomes for synthesizing some of the mitochondrial proteins that carry out oxidative phosphorylation to produce the cell’s ATP. Although mitochondria are thought to have descended from symbiotic bacteria, their ribosomes differ substantially from those of modern bacteria. In particular, the ratio of protein to rRNA in animal mitochondrial ribosomes is 2:1 by mass, rather than the 1:2 ratio observed for bacterial and archaeal ribosomes. Because the ribosomes have roughly the same mass, this means that mitochondrial ribosomes are two-thirds protein and one-third rRNA by mass, whereas bacterial ribosomes are one-third protein and two-thirds rRNA. What does this imply about the origins of the ribosome and the importance of rRNA in its function?
To begin to answer these questions, Rajendra Agrawal and Stephen Harvey proposed a structural model of the mitochondrial large ribosomal subunit, using a combination of cryo-electron microscopy and molecular modeling. Although the resolution of the electron microscopy–derived electron density, 12.1 Å, was lower than that of x-ray crystallography, it was possible to model higher-resolution bits of structure into the electron density map by using various molecular landmarks. The resulting model predicts the arrangement of individual protein and rRNA components in the large subunit of the mitochondrial ribosome (Figure 1). Although there is much less rRNA than r-protein, the rRNA forms the same overall architecture and occupies the same positions known to be essential for protein synthesis in bacterial and archaeal ribosomes. However, the r-proteins encroach on the all-RNA core of the mitochondrial ribosome, where they substitute for segments of rRNA that are missing relative to bacterial ribosomes.
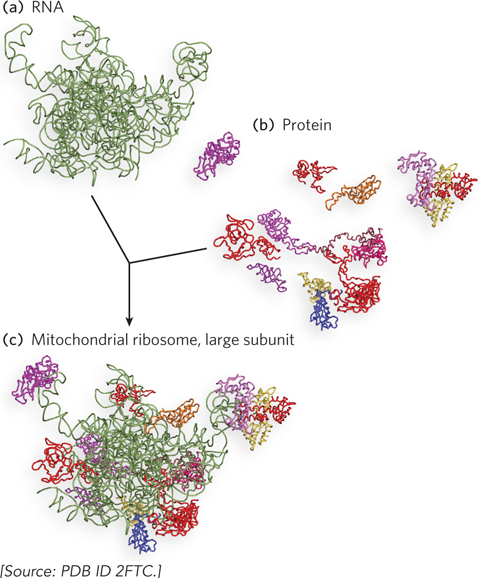
FIGURE 1 (a) The RNA portions and (b) the protein portions of the large ribosomal subunit of mammalian mitochondria, determined by cryo-electron microscopy and modeled using available crystal structures of the bacterial 50S ribosomal subunit. (c) The proteins and RNAs making up the complete large subunit. Notice how protein segments penetrate the interior of the subunit.
Because mtDNA has a higher mutation rate and therefore evolves faster than the cellular genome, the mitochondrial ribosome presumably is now farther from its all-RNA origins than is the bacterial ribosome. This may reflect the greater structural and functional diversity of proteins relative to RNA. Far in the future, mitochondrial and, eventually, all ribosomes might consist primarily of protein, as their rRNA components are replaced over time. Mammalian mitochondria have evolved to synthesize just 13 proteins, so their ribosomes may be subject to different selective pressures from those at work on cytosolic ribosomes. Comparative studies of mitochondrial and cytosolic ribosome structure and activity may provide unexpected insights into the changing roles of RNA and protein in ribosome form and function.
The Ribosome Structure Facilitates Peptide Bond Formation
The ribosome must bind simultaneously to at least two tRNAs during each cycle of amino acid addition to the C-terminus of a growing polypeptide chain. In fact, experiments to measure tRNA interactions on the ribosome, as well as the parts of tRNA molecules that are protected from solvent when bound to the ribosome, showed that ribosomes contain binding sites for three tRNAs (Figure 18-8). The A site is the location of aminoacyl-tRNA binding, the P site is the location of peptidyl-tRNA binding, and the E site is the exit site, occupied by the tRNA molecule released after the growing polypeptide chain is transferred to the aminoacyl-tRNA. Each tRNA starts in the A site, moves to the P site after peptide bond formation, then exits through the E site.
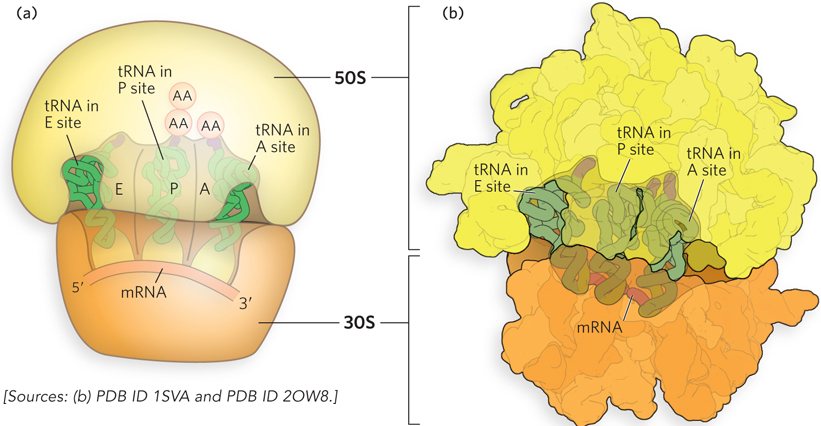
Figure 18-8: The A, P, and E sites of the ribosome. (a) The A, P, and E sites in relation to the bound mRNA. Both ribosomal subunits are shown. AA indicates an amino acid bound to tRNA. (b) A representation of the E. coli 70S ribosome crystal structure with bound tRNAs—representing aminoacyl-tRNA, peptidyl-tRNA, and free tRNA, respectively—in the A, P, and E sites, viewed from the 30S interface.
Each of these sites spans the two ribosomal subunits and thereby functionally links the decoding center of the small subunit with the peptidyl transferase center of the large subunit. The anticodon loop at one end of each L-shaped tRNA molecule makes contact with the mRNA positioned in the small-subunit decoding site, and the aminoacylated 3′ end of the tRNA occupies the peptidyl transferase center in the large subunit, 70 Å away.
During elongation, the ribosome houses a growing polypeptide chain, which is covalently linked to the tRNA in the P site, as the peptidyl-tRNA. Each time the ribosome shifts from one mRNA codon to the next, it makes room for a new aminoacyl-tRNA in the A site. After the peptide bond forms (catalyzed by the peptidyl transferase), the polypeptide chain transfers from one tRNA to the other, with the aminoacyl-tRNA becoming the peptidyl-tRNA as the growing polypeptide chain is added to it.
The rate of peptide bond formation is enhanced on the ribosome largely because the 3′ ends of the two reacting tRNAs are positioned optimally for the chemical reaction to occur (Figure 18-9a). Note that there is no accompanying hydrolysis of a high-energy bond, such as that of a nucleoside triphosphate, at this stage of protein synthesis. This is because each amino acid has already been activated by its attachment to tRNA in an aminoacylation reaction. As we will see, the aminoacylation step requires ATP hydrolysis, so the energy cost has already been paid (see Section 18.2). Thus, each acyl group provides the high-energy bond that is hydrolyzed to drive the formation of a new peptide bond during the peptidyl transferase reaction.
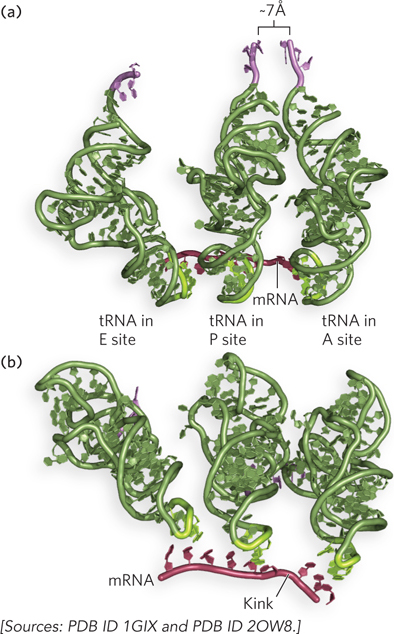
Figure 18-9: Alignment of tRNAs on mRNA and the peptidyl transferase site. (a) The peptidyl-tRNA and the aminoacyl-tRNA are positioned so that the amino acids are at the optimal distance for peptide bond formation. (b) The anticodons kink the mRNA.
Crystal structures of the ribosome revealed the presence of channels for the movement of mRNAs and polypeptides during protein synthesis. In the small subunit, the mRNA entry and exit channels are narrow clefts with space to accommodate only a single strand of RNA. Thus, an mRNA must be unfolded and available for base pairing to tRNAs when it enters the decoding center. The A and P sites in the small subunit provide room for tRNAs to base-pair with two adjacent codons in the mRNA sequence. Here, the ribosome induces a kink in the mRNA between the two codons to help ensure accuracy of the reading frame (see Figure 18-9b). This kink may also contribute to correct positioning of the aminoacylated ends of the tRNA in the peptidyl transferase center of the large subunit.
In the large subunit, a tunnel (50 Å long) allows the exit of polypeptides during translation (Figure 18-10). The diameter of the exit tunnel seems to preclude formation of structures wider than an α helix as the polypeptide is being synthesized. Thus, nascent proteins lack tertiary structure and must assemble into their correct three-dimensional structure after exiting the ribosome.
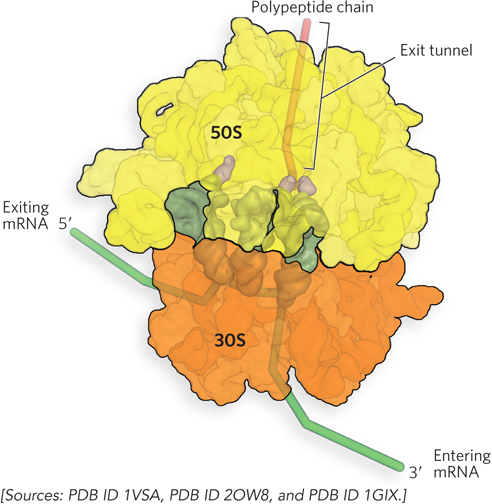
Figure 18-10: The exit tunnel for protein in the 50S subunit. The protein exit tunnel is adjacent to the P site and only wide enough to allow an α helix or an unfolded polypeptide to pass through.
SECTION 18.1 SUMMARY
Protein synthesis occurs on ribosomes, large complexes of protein and rRNA. Bacteria have 70S ribosomes, with a large (50S) and a small (30S) subunit. Eukaryotic ribosomes are significantly larger, 80S (with 60S and 40S subunits), and contain more proteins.
Protein synthesis is regulated by the ability of the small ribosomal subunit to associate independently with the mRNA before translation begins. Once the ribosome is fully assembled, it moves along the mRNA, matching a tRNA to each codon and catalyzing peptide bond formation. Multiple ribosomes can occupy a single mRNA, forming a polysome.
In all organisms, the rRNA of the large ribosomal subunit catalyzes peptide bond formation. The genetic code is read on the small ribosomal subunit, which ensures that the correct amino acid is added to the growing polypeptide chain.
Aminoacyl-tRNAs first bind to the A site of the ribosome. As the peptide bond is formed between the amino acid and the growing peptide chain, the newly formed peptidyl-tRNA moves to the P site and the free tRNA exits through the E site.
The mRNA and growing polypeptide pass through separate channels in the ribosome that require them to be unfolded. The ribosome induces a kink in the mRNA between the A and P sites to allow base-pairing of the tRNAs. Polypeptides form their functional three-dimensional structure after emerging from the ribosomal exit tunnel.













