CONCEPT34.3 Neurons Communicate with Other Cells at Synapses
Recall that synapses are cell-to-cell contact points that are specialized for signal transmission from one cell to another. They play extremely important roles in information processing and integration. They also play central roles in learning and memory. A great deal of modern research on the function of the nervous system focuses on synapses.
Chemical synapses are most common, but electrical synapses also exist
The most common type of synapse is termed a chemical synapse because signals pass from cell to cell by means of molecules (“chemicals”) released by the presynaptic cell. At a chemical synapse, the cell membranes of the pre- and postsynaptic cells are actually separated by an extremely narrow space, about 20–30 nm wide, termed the synaptic cleft. Action potentials cannot cross this space (although the space is bridged by occasional trans-synaptic protein molecules). When an action potential arrives at the end of the presynaptic cell, that cell releases a compound called a neurotransmitter into the synaptic cleft, and the neurotransmitter crosses the cleft. Acetylcholine is an example of a neurotransmitter:
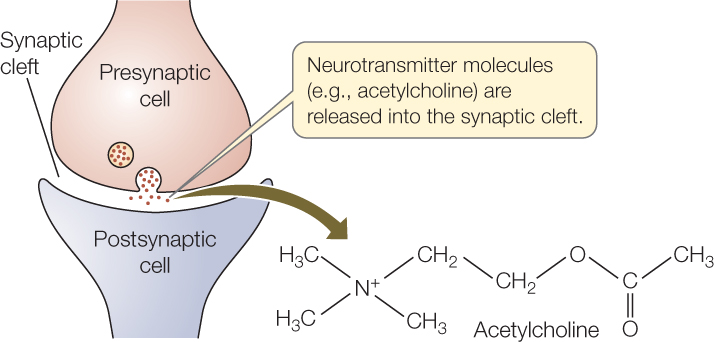
Neurotransmitter molecules diffuse across the synaptic cleft very rapidly because the distance is extremely short. The molecules then bind to receptor proteins in the cell membrane of the postsynaptic cell. These receptor molecules are specialized to generate an electrical change or some other sort of change when they bind to neurotransmitter molecules—completing signal transmission across the synapse. In many cases, the entire process of synaptic signal transmission takes just a few milliseconds.
Neurotransmitter molecules are rapidly removed from the synaptic cleft after they have been released into it by the presynaptic cell. Removal terminates the signal and is essential for the proper function of chemical synapses. Removal may occur by two principal mechanisms. The neurotransmitter molecules may be enzymatically broken down, or they may be taken up by nearby neurons or glial cells. In some well-known cases, neurotransmitter molecules are removed from the synapse by reuptake into the presynaptic cell.
In an electrical synapse, signal transmission is “all electrical” instead of employing a neurotransmitter. At such a synapse, the cell membranes of the pre- and postsynaptic cells are joined by gap junctions at which the cytoplasms of the two cells are continuous (see Figure 4.18). Thus when an action potential arrives in the presynaptic cell, it can spread easily and directly to the postsynaptic cell. Signals cross electrical synapses with essentially no delay.
Electrical synapses have evolved principally where very fast, invariant signal transmission is advantageous. They are found in neurons that control escape swimming in some fish and crustaceans. They are also found in some tissues where great numbers of cells must be stimulated synchronously to act together, such as fish electric organs (see Concept 33.4).
The vertebrate neuromuscular junction is a model chemical synapse
Each muscle cell (muscle fiber) in a vertebrate skeletal muscle is typically innervated by one neuron, called a motor neuron. A muscle such as your biceps contains thousands of muscle cells, and many neurons are involved in innervating the whole muscle. However, each muscle cell is typically innervated by just one neuron, and the muscle cell will contract only if stimulated to do so by that neuron.
A neuromuscular junction—a type of chemical synapse—is a synapse between a motor neuron and a skeletal muscle cell (FIGURE 34.9A). This type of synapse, sometimes called a motor end-plate, has been studied intensively to understand how chemical synapses work. It is not entirely representative of all chemical synapses because it is specialized for a one-to-one relationship between nerve impulses and muscle contractions: each action potential in the presynaptic neuron depolarizes the postsynaptic (muscle) cell to threshold and produces an action potential in the postsynaptic cell. Chemical synapses in the brain do not operate this way, as we will discuss later in this chapter.
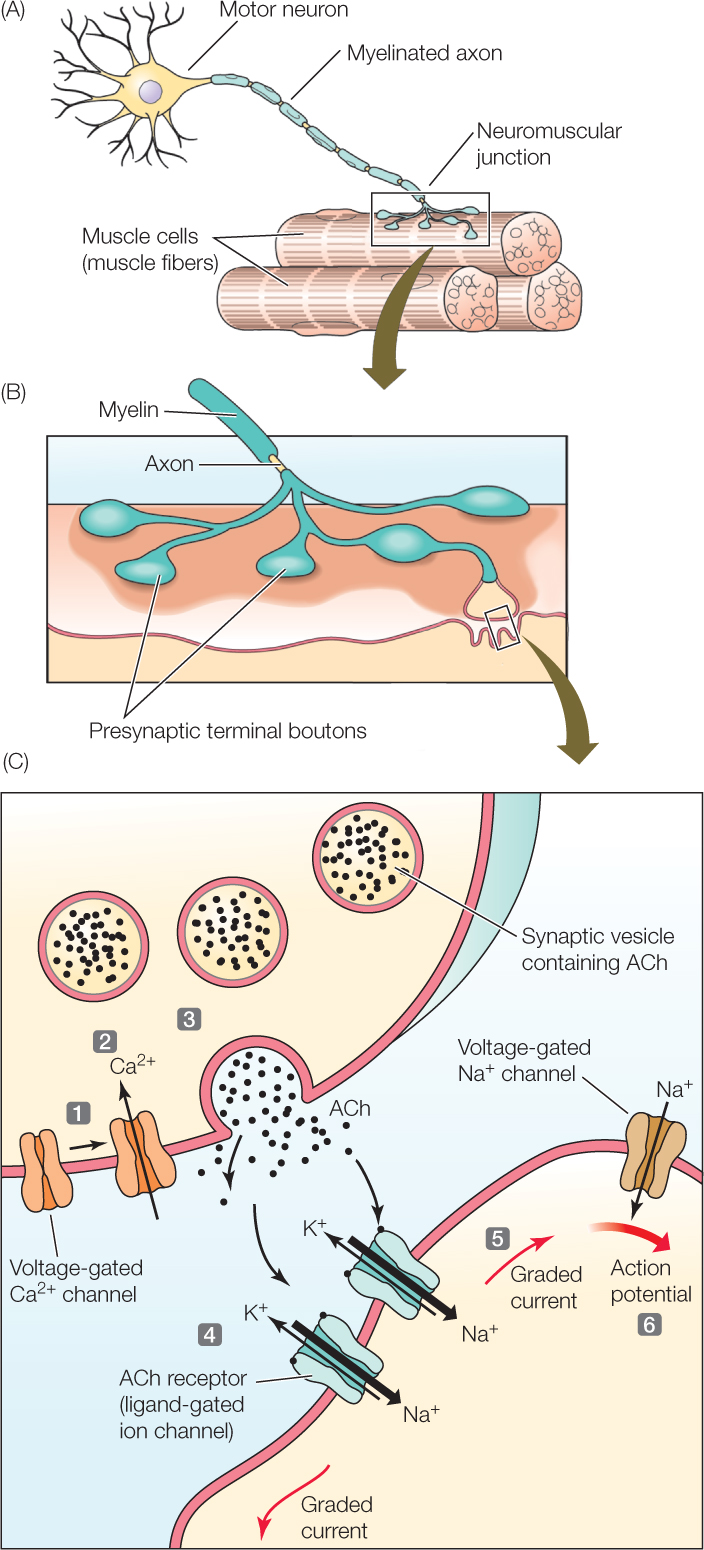
At a neuromuscular junction, the axon of the presynaptic neuron branches close to the muscle cell, creating several buttonlike axon terminals (called boutons) that are in synaptic contact with the muscle cell (FIGURE 34.9B). Each of these axon terminals contains spherical synaptic vesicles filled with molecules of acetylcholine (ACh), the neurotransmitter used by all vertebrate neuromuscular junctions (FIGURE 34.9C).
When an action potential arrives at an axon terminal, the terminal releases ACh into the synaptic cleft by a mechanism that depends on voltage-gated calcium channels. The action potential causes Ca2+ channels in the presynaptic membrane to open (see Figure 34.9C, step 1). Ca2+ diffuses into the axon terminal through the open channels because the concentration of Ca2+ is greater outside the cell than inside (step 2). Increased Ca2+ inside the terminal induces synaptic vesicles to fuse with the presynaptic membrane, releasing ACh into the synaptic cleft by exocytosis (step 3; see Figure 5.8B).
Neurons communicate with other cells at synapses
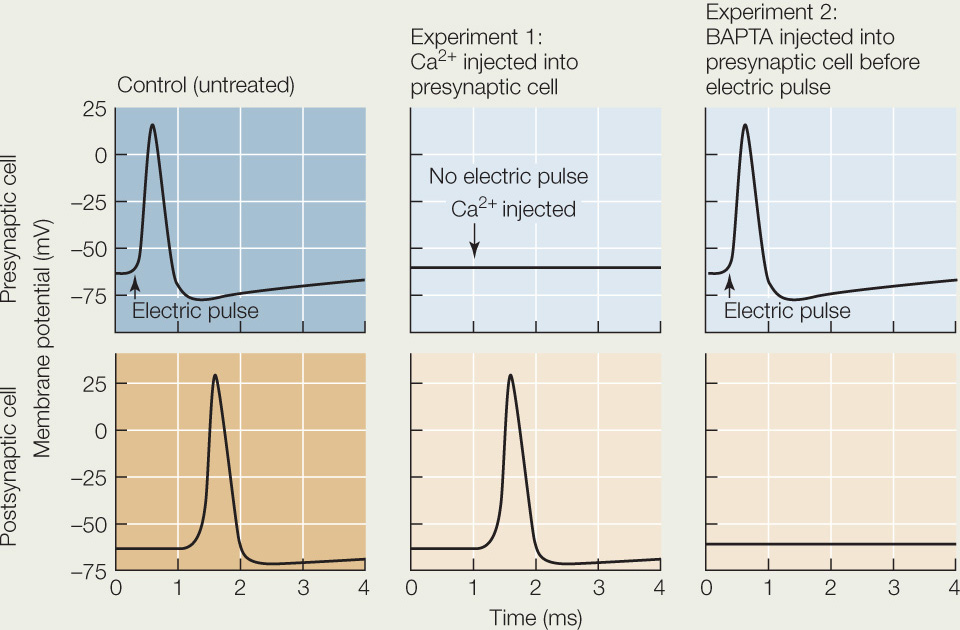
How do we know that Ca2+ influx into the presynaptic neuron ending causes the release of neurotransmitter? The squid giant axon and its neuron endings are a convenient system for experiments because they are so large, making it possible to inject substances into the presynaptic cell at a synapse. Some of the substances that can be injected are Ca2+ ions and BAPTA, a substance that binds Ca2+ ions. Shown below are the results of a series of experiments using these substances.
- What is happening during the delay between the pre- and postsynaptic membrane events in the control condition?
- Explain the postsynaptic response in the absence of a presynaptic electric signal in experiment 1.
- Explain why there is a presynaptic but no postsynaptic response in experiment 2.
The response of the muscle cell depends on ligand-gated receptors in the postsynaptic cell membrane at the synapse (see Figure 34.5C). These receptors, which are gated by ACh, act as ion channels when they are open. When ACh is released into the synaptic cleft, it binds to the ACh-gated receptor proteins (step 4). These receptors open, allowing Na+ and K+ to diffuse through. Influx of Na+ is dominant because of the locally prevailing ion and electrical gradients. The rapid influx of Na+ causes a large, graded depolarization, which induces graded ionic currents that spread to neighboring regions of the postsynaptic membrane (step 5) where there are voltage-gated Na+ and K+ channels. The depolarization in these neighboring regions is great enough to exceed the threshold of the voltage-gated ion channels, setting off an action potential (step 6). This action potential is propagated throughout the cell membrane of the muscle cell, and it propagates into the cell’s transverse tubules (T tubules), activating contraction.
LINK
The physical and molecular interactions of muscle contraction are described in Concept 33.1
Many neurotransmitters are known
Several dozen neurotransmitters are now known, and more are being discovered all the time. They fall into three major chemical categories:
- Amino acids that serve as neurotransmitters include glutamate, glycine, and γ-aminobutyric acid (GABA; γ = gamma).
- Biogenic amines include acetylcholine and also dopamine, norepinephrine, and serotonin.
- A variety of peptides (strings of amino acids) serve as neurotransmitters.
Each presynaptic neuron produces specific neurotransmitters. Some produce only one, and others produce two or more, but all presynaptic neurons are specific in the neurotransmitters they release. For simplicity, we’ll assume as we go forward that each neuron releases one neurotransmitter.
Let’s now consider a neuron that is functioning as the postsynaptic cell. In the brain, a postsynaptic neuron may have chemical synapses with hundreds or thousands of presynaptic neurons (FIGURE 34.10). Each of these neurons will use one neurotransmitter. However, different neurons may use different neurotransmitters. Collectively, the hundreds or thousands of presynaptic cells may use dozens of neurotransmitters. Moreover, the receptor proteins for any particular neurotransmitter in the cell membrane of the postsynaptic cell may be of two or more molecular types that have different actions. The action of a neurotransmitter at any particular synapse ultimately depends on the action of the specific receptor to which it binds there.
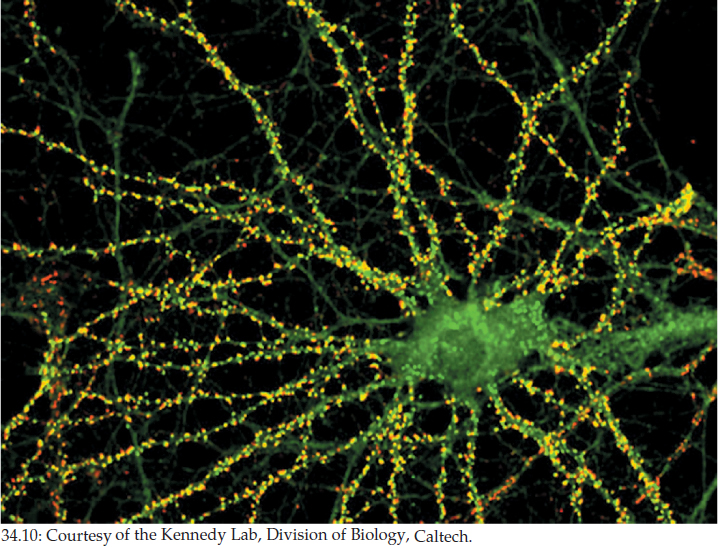
This complexity in synapse function helps us understand how brain function can be as complex as it is. The model chemical synapse we discussed, the neuromuscular junction, employs one neurotransmitter and one type of receptor. Modern brain scientists, however, seek to understand neurons that have great numbers of synapses that function with a variety of neurotransmitters, and often with a variety of receptor proteins for any given neurotransmitter.
Synapses can be fast or slow depending on the nature of receptors
Two broad classes of neurotransmitter receptors are recognized. These are fast, ionotropic receptors and slow, metabotropic receptors.
Ionotropic receptors are ligand-gated ion channels similar to the ACh receptor in the vertebrate neuromuscular junction. Neurotransmitter binding to an ionotropic receptor directly causes a fast, short-lived change in ion diffusion across the cell membrane of the postsynaptic cell. In this way it can directly cause an immediate change in the local membrane potential. This change may in turn initiate other changes in the postsynaptic cell.
Metabotropic receptors are not ion channels. Instead, they are commonly G protein–linked receptors (see Figure 5.14). Such receptors often exert their effects by controlling production of second messengers such as cyclic adenosine monophosphate (see Figure 5.17). Recall that second messengers carry messages to the interior of a cell, where they can exert controlling effects on a wide variety of cell mechanisms, and even on gene expression. Postsynaptic cell responses mediated by metabotropic receptors are very diverse and are often slow and long-lived.
LINK
The structure and properties of G protein–linked receptors and the cellular responses they activate are described in Concept 5.5 and Concept 5.6
Fast synapses produce postsynaptic potentials that sum to determine action potential production
In Concept 34.2 we discussed the fact that graded depolarizations and hyperpolarizations of a cell membrane sum together to determine if the voltage threshold for action potential production is reached. This process is a key mechanism by which single neurons integrate their inputs. Let’s now review the process with specific information on synaptic function.
At neuron-to-neuron synapses, when a postsynaptic ligand-gated ion channel opens, the change caused in the membrane potential of the postsynaptic cell is usually less than 1 mV! This is very different from what happens at a neuromuscular junction. The neuromuscular junction is specialized so that each presynaptic nerve impulse depolarizes the postsynaptic cell sufficiently for an action potential to be generated. Neuron-to-neuron synapses, by contrast, are specialized so that each presynaptic impulse produces just a fast, small, short-lived postsynaptic effect. The exact nature of the effect depends on the neurotransmitter employed and on the receptor protein, including which ion fluxes are altered.
- Synapses that depolarize the postsynaptic membrane are called excitatory synapses. They are so named because depolarization shifts the membrane potential toward the threshold for action potential production, increasing the likelihood that an action potential will be triggered. Excitatory synapses produce graded membrane depolarizations called excitatory postsynaptic potentials (EPSPs).
- Synapses that shift the membrane potential away from threshold are called inhibitory synapses because they lower the likelihood of action potential production. Inhibitory synapses produce graded membrane hyperpolarizations called inhibitory postsynaptic potentials (IPSPs).
Each individual EPSP or IPSP, produced at a single time by a single synapse, is usually less than 1 mV, as we have said, and disappears in 10–20 milliseconds. The EPSPs and IPSPs are typically produced at dendrites and at the cell body (the dendrites and cell body are the sites of the synapses).
EPSPs and IPSPs, being graded potentials, fade as they spread along the cell membrane of a postsynaptic neuron. When they spread, however, they affect the membrane potential in the part of the neuron near the axon hillock where action potentials are initiated. Membrane potentials must be present at a single time to sum together. They may originate from many spatially separate synapses (FIGURE 34.11). Summation is thus both temporal (dependent on time) and spatial. Keep in mind that each individual EPSP or IPSP lasts only 10–20 milliseconds. Thus the sum of the graded potentials changes from moment to moment.
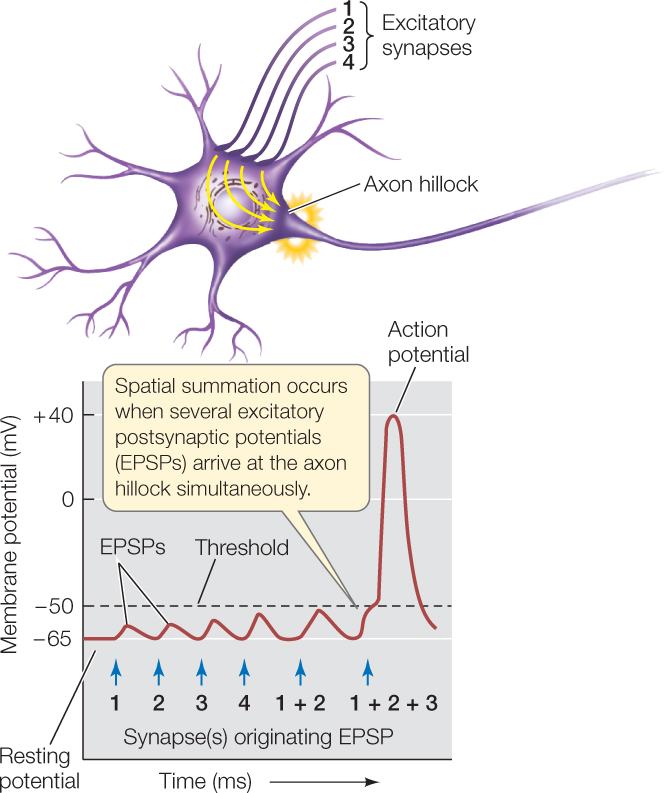
Summation determines whether the postsynaptic cell produces action potentials. If the sum of EPSPs and IPSPs at the axon hillock is a depolarization great enough to reach threshold, the cell produces an action potential. If the depolarization is greater than threshold after the refractory period, the cell produces another action potential. In this way, a long period of depolarization above threshold results in a long sequence of closely spaced action potentials.
Synaptic plasticity is a mechanism of learning and memory
Synaptic plasticity is a phenomenon of extreme importance that is a principal focus of modern synapse research. Synaptic plasticity refers to the fact that synapses in the nervous system of an individual animal can undergo long-term changes in their functional properties and even their physical shape during the individual’s lifetime. Such changes are currently hypothesized to be one of the major mechanisms of learning and memory.
Learning refers to the ability of an individual animal to modify its behavior as a consequence of individual experiences that took place at earlier times. For instance, if we have been accustomed to nibbling mushrooms while walking in the woods but one day we become deathly ill after doing so, we will change our behavior in the future: seeing mushrooms, we will no longer grab one for a snack. What is the mechanism by which such learning might occur? Synaptic plasticity is one hypothesis. According to this hypothesis, an individual’s experiences at one time in its life can produce long-term changes in the individual’s synapses, so that future experiences are then processed by the nervous system in altered ways.
LINK
Some of the many ways animal behavior is modified by learning and early experiences are described in Concept 40.2
Two model examples of synaptic plasticity illustrate its mechanisms and importance. Sea hares (marine mollusks in the genus Aplysia) pull their gills inside when certain parts of their body are touched:
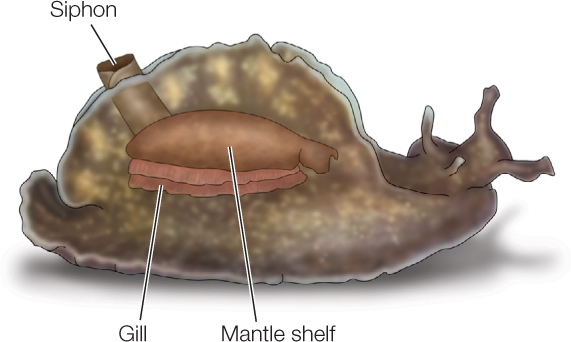
The sea hares learn from past experience how vigorously to do this. They withdraw their gills more vigorously if they have previously been exposed to a very noxious agent—a type of learning called sensitization. Sensitization occurs because of synaptic plasticity in synapses between the sensory neurons that detect touching and the motor neurons that stimulate the gill-withdrawal muscles. These synapses are said to be functionally strengthened when a sea hare is subjected to a noxious stimulus: the synapses become altered so that the amount of neurotransmitter released per presynaptic signal is increased, causing the postsynaptic cell to be excited to a greater degree by an identical presynaptic input. Long-term changes brought about by a second-messenger system are responsible.
Studies of the mammalian hippocampus provide a vivid example of synaptic plasticity in which synapses change not only in function but also in physical characteristics. The hippocampus lies deep within the brain:
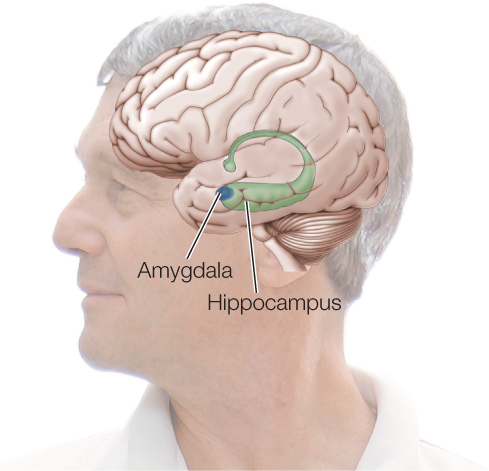
It is involved in spatial learning and memory formation. Studies have been carried out on living hippocampal tissue slices isolated from fully mature mice. Certain neuronal circuits can be stimulated in these preparations while neighboring circuits are not. Investigators have shown that when a circuit is repeatedly stimulated, certain of the postsynaptic structures in that particular circuit physically grow. Along with this physical response, the synapses also strengthen functionally. In this case, the number of active postsynaptic receptor molecules in each postsynaptic cell increases. Consequently, the cell responds more strongly to synaptic input from the presynaptic cell. Synaptic plasticity in this case has been shown to depend on second messengers, altered protein synthesis, and altered gene transcription in the postsynaptic cells. If you imagine some information being processed by circuits that are identical except that some have received a lot of prior stimulation and others have not, it is clear that the previously stimulated circuits will process the information differently, providing a basis for both learning and memory.
CHECKpointCONCEPT34.3
- At a vertebrate neuromuscular junction, what would happen if a poison blocked the mechanism that removes acetylcholine from the synapse after it has had its usual effect?
- Consider the arrival of a single excitatory presynaptic signal at a single chemical synapse. What is the difference between the magnitude of the postsynaptic graded potential at a vertebrate neuromuscular junction and at a neuron-to-neuron synapse, and why does it matter?
- What is meant by synaptic plasticity, and speaking very generally, how could it be a mechanism of learning?
We have seen how neurons use ion pumps and ion channels to generate action potentials, and how action potentials trigger neurotransmitter release, allowing neurons to communicate with each other and with other cells. Next we’ll look at how systems of neurons can receive sensory information.