13-2 Neural Basis of the Biological Clock
Curt Richter (1965) was the first researcher who attempted to locate biological clocks in the brain. In the 1930s he captured wild rats and tested them in activity wheels. He found that the animals ran, ate, and drank when the lights were off and were relatively quiescent when the lights were on.
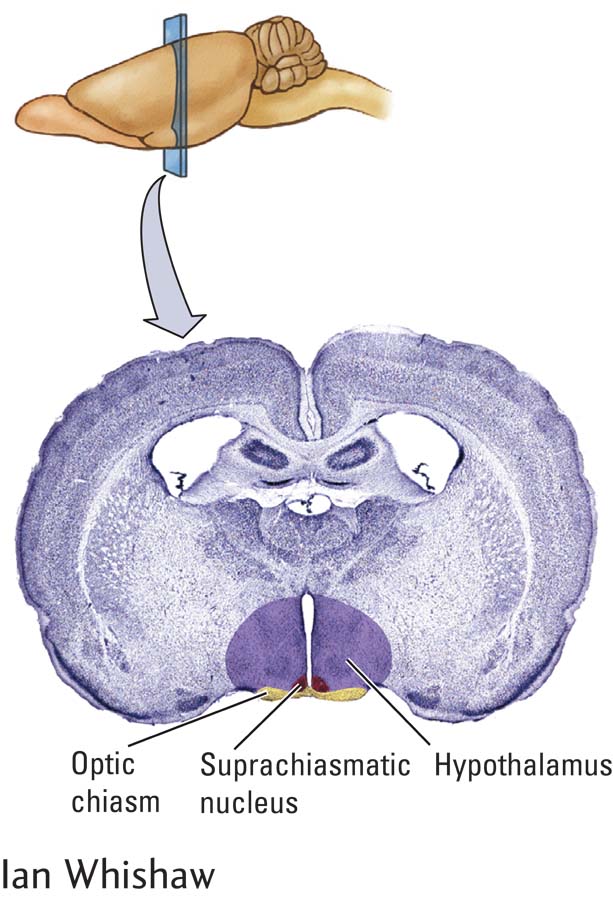
By ablating brain tissue with electric current, Richter found that animals lost their circadian rhythm after damage to the hypothalamus. Subsequently, by making much more discrete lesions, experimenters have shown that a region of the hypothalamus, the suprachiasmatic nucleus (SCN), acts as the master biological clock (Webb et al., 2014). The SCN is named for its location just above (supra-
Suprachiasmatic Rhythms
Evidence for the SCN’s role in circadian rhythms now comes from additional lines of evidence:
If the suprachiasmatic nuclei are selectively damaged, animals still eat, drink, exercise, and sleep but at haphazard times.
If a form of glucose is tagged with a radioactive label, taken up by metabolically active cells, and trapped in them but not used by them, cells that are more active will subsequently emit more radioactivity. When this tracer is injected into rodents, more is found in the SCN after injections given in the light period of the light–
dark cycle than in the dark period. This experiment demonstrates that suprachiasmatic cells are more active during the light period. Recording electrodes placed in the SCN confirm that neurons in this region are more active during the light period of the cycle than during the dark period.
If all the pathways into and out of the suprachiasmatic nucleus are cut, SCN neurons maintain their rhythmic electrical activity.
SCN cells removed from the brain and cultured in a dish retain a periodic rhythm.
Clearly, suprachiasmatic neurons have an intrinsically rhythmic activity pattern. Although the SCN is the master biological clock, it is not the sole one. Two other neural structures, the intergeniculate leaflet and the pineal gland, also display clocklike activity. Further, nearly every cell in the body has its own clock.
After the SCN is destroyed, some behaviors retain a timed occurrence. One is feeding. Animals without an SCN can still display anticipatory behavior—
Keeping Time
GABA is main inhibitory neurotransmitter in the CNS.
If SCN neurons are isolated from one another, each remains rhythmic, but the period of some cells differs from that of other cells. Thus rhythmic activity is a property of SCN cells, but the timing of the rhythm must be set so that the cells can synchronize their activity in relation to each other. In the brain, SCN cells connect one to another through inhibitory GABA synapses, and these connections allow them to act in synchrony. Their entrainment depends upon external inputs, however.
Section 9-2 traces three main routes from the retina to the visual brain. Figure 9-8 diagrams the retina’s cellular structure.
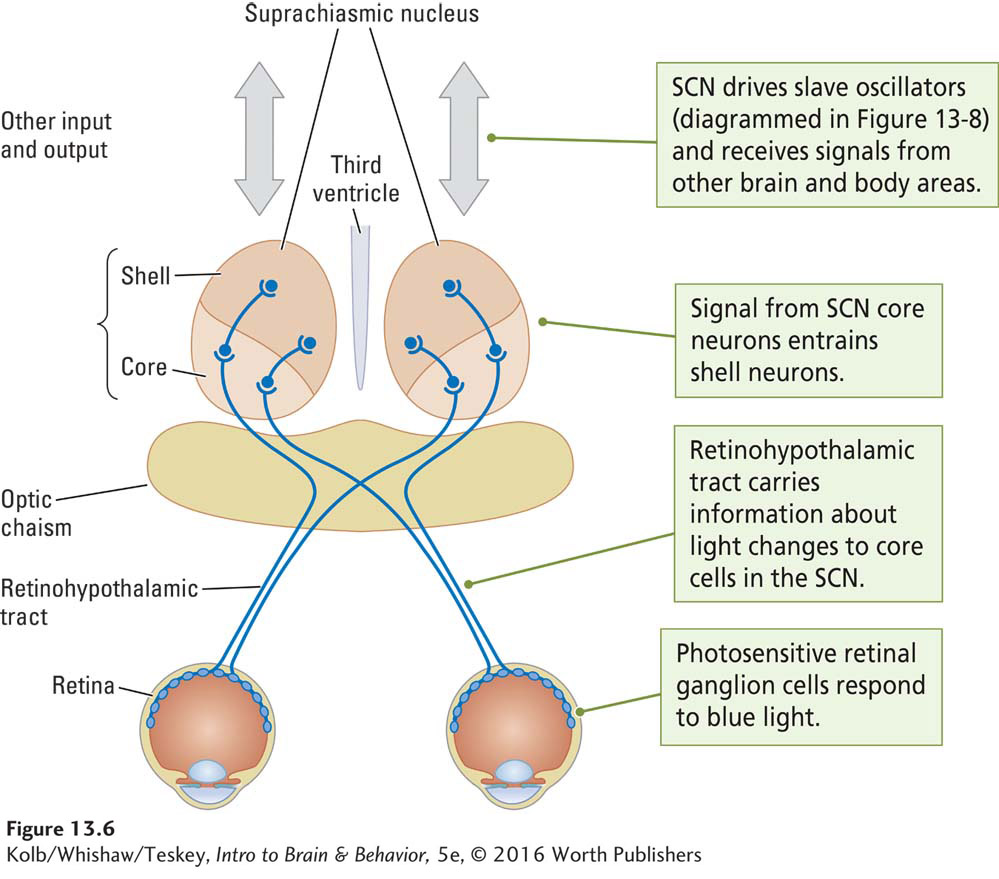
The SCN receives information about light through the retinohypothalamic tract (Figure 13-6). This pathway begins with specialized retinal ganglion cells (RGCs) that contain the photosensitive pigment melanopsin. These melanopsin-
Glutamate is the main excitatory neurotransmitter in the CNS.
Melanopsin-
When stimulated by light, melanopsin-
As illustrated in Figure 13-6, the SCN consists of two parts, a more ventrally located core and a more dorsally located shell. The retinohypothalamic tract activates the core cells. Core neurons are not rhythmic, but they entrain the shell neurons, which are rhythmic.
In addition to retinohypothalamic input, the SCN receives projections from other brain regions, including the intergeniculate leaflet in the thalamus and the raphe nucleus, which is the nonspecific serotonergic-
The SCN’s circadian rhythm is usually entrained by morning and evening light, but it can also be entrained or disrupted by sudden changes in lighting, by arousal, by moving about, and by feeding. These influences differ from the light entrainment provided over the retinohypothalamic tract.
The intergeniculate leaflet and the raphe nucleus are pathways through which nonphotic events influence the SCN rhythm (Cain et al., 2007). The neural structures mediating these other entraining pathways explain why being aroused or eating during the sleep portion of the circadian cycle disrupts the cycle (Mistlberger & Antle, 2011).
It is likely that the regional variation in SCN shell anatomy provides the substrate for various rhythms of the circadian cycle. Findings from studies on the genes that control rhythms in fruit flies suggest two separate groups of circadian neurons. M cells control morning activity; they need morning light for entrainment. E cells control evening activity; they need onset of darkness for entrainment (Hughes et al., 2015).
Some people are early to bed and early to rise and are energetic in the morning. Other people are late to rise and late to bed and are energetic in the evening. Individual differences in circadian activity between these “lark” and “owl” chronotypes are due to differences in SCN shell neurons. The differences may be the equivalent of fruit fly M cells and E cells, When expressed differently in people, genes may account for differences in the amplitude of phases in the circadian period. (Pellegrino et al, 2015). Whether students are larks or owls does affect their performance on cognitive tasks, possibly by affecting memory for content (Smarr, 2015).
In hamsters and mice, mutant gene variations produce chronotypes with circadian periods as varied as 24, 20, or 17 hours (Monecke et al., 2011). As genetic analysis becomes less expensive, the study of the genes underlying human chronotypes is providing insights into individual differences in our biological clocks (Kang et al., 2015).
Immortal Time
How do suprachiasmatic cells develop rhythmic activity? Their endogenous rhythm is not learned. When animals are raised in constant darkness, their behavior still becomes rhythmic. In experiments in which animals have been maintained without entraining cues for a number of generations, each generation continues to display rhythmic behavior. Even if the mother has received an SCN lesion so that her behavior is not rhythmic, her offspring’s behavior is rhythmic.
A line of evidence supporting the idea that suprachiasmatic cells are genetically programmed for rhythmicity comes from studies performed in Canada by Martin Ralph and his coworkers with the use of transplantation techniques (Ralph & Lehman, 1991). Figure 13-7 illustrates the experiment.
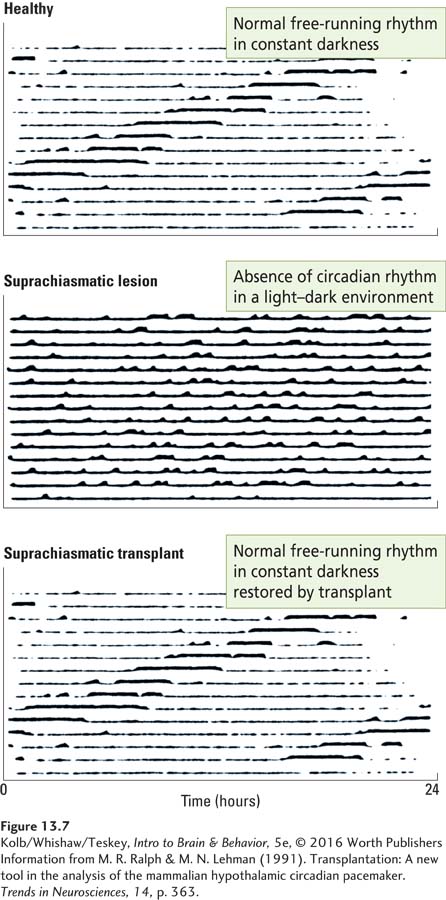
As reflected in the top chart, hamsters are tested in constant dim light or in constant darkness to establish their free-
What Ticks?
Molecular research is directed toward determining what genes control the ticking of the circadian clock. The timing device is in each SCN neuron and in most other cells of the body as well.
Figure 3-13 diagrams the process of protein synthesis.
The circadian rhythm involves a feedback loop in which proteins are first made and then combine. The combined protein, called a dimer, for two proteins, inhibits production of its component proteins. Then the dimer degrades and the process begins anew. Research Focus 13-3, Synchronizing Biorhythms at the Molecular Level, describes the main feedback loop in mammals. Just as the back-
Pacemaking Circadian Rhythms
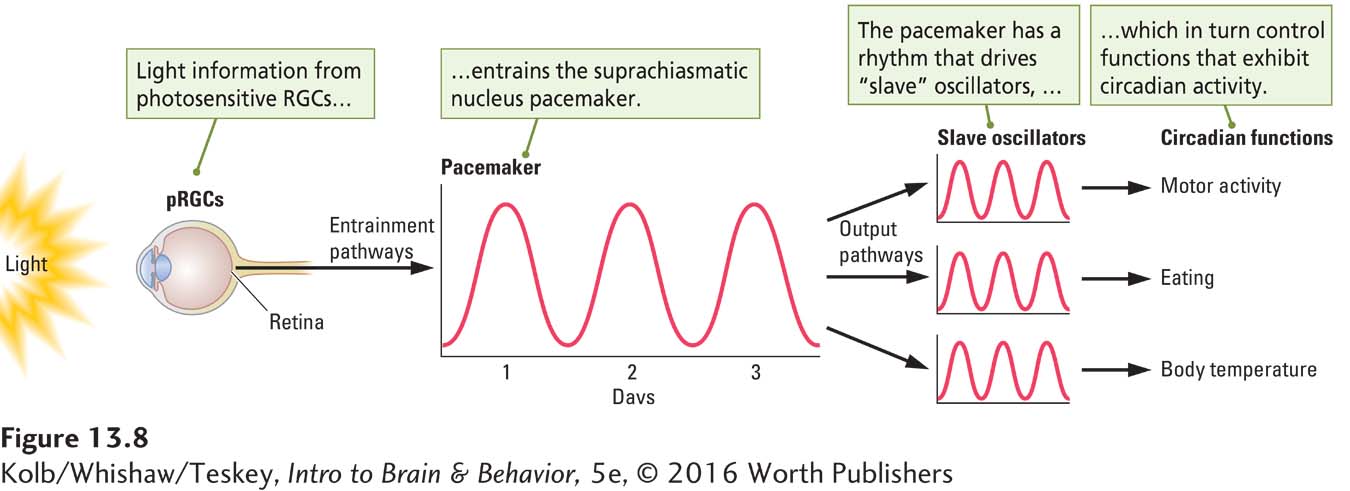
The SCN itself is not directly responsible for producing behavior. After it has been damaged, drinking and eating, sleeping and wakefulness still occur. They no longer occur at appropriate times, however.
An explanation for how the SCN controls biological rhythms is illustrated in Figure 13-8. In this model, light entrains the SCN, and the SCN in turn drives a number of slave oscillators. Each slave oscillator is responsible for the rhythmic occurrence of one activity. In other words, drinking, eating, body temperature, and sleeping are each produced by a separate slave oscillator.
13-3
Synchronizing Biorhythms at the Molecular Level
The retinohypothalamic tract carries information about light changes from the melanopsin-
The timing mechanism in the shell cells is a pair of interlocking feedback loops that pace the SCN’s function over a 24-
The main clock mechanism is the transcription-
STEP 1: TRANSCRIPTION In the cell nucleus three Period genes (Per1,* Per2, Per3) and two Cryptochrome genes (Cry1, Cry2) are transcribed into Per1, Per2, and Per3 messenger RNA and Cry1 and Cry2 mRNA.
STEP 2: TRANSLATION Ribosomes in the endoplasmic reticulum translate these mRNAs into the proteins PER1,† PER2, PER3, and CRY1, CRY2. In the intracellular fluid the proteins form various dimers, or two-
STEP 3: INHIBITION PERCRY dimers enter the cell nucleus, where they bind to and inhibit the CB dimer (formed by the CLOCK and BMAL proteins). The CB dimer turns on the Enhancer box (Ebox), a part of the DNA that activates transcription of the Period and Cryptochrome genes, so that when the CB dimer is inhibited, the Per and Cry genes are no longer expressed.
STEP 4: DECAY After they play their inhibitory role, the PERCRY proteins decay. Then the CB dimer resumes its activity, the Per and Cry genes resume expression, and the 24-
This sequence of gene turn-
Mutations in any circadian gene can lead to circadian alterations, including absence of a biorhythm or an altered biorhythm. For example, alleles of Period 1 and Period 2 genes determine chronotype—
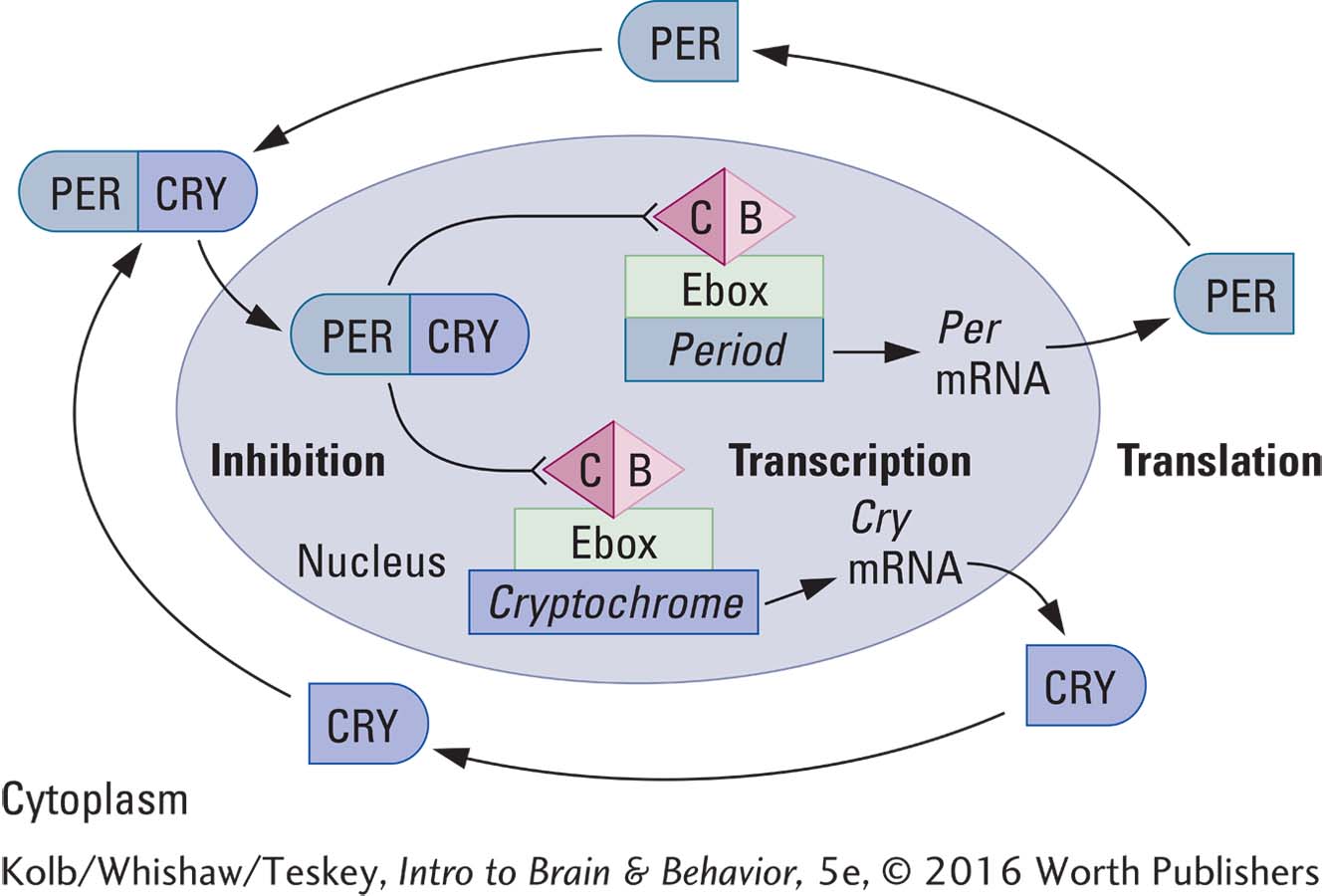
*Gene and mRNA names are italicized.
†PROTEIN names are capitalized.
The SCN clock entrains slave oscillators through a remarkable array of pathways:
SCN neurons send axonal connections to nuclei close by in the hypothalamus and thalamus. These nuclei in turn connect extensively with other brain and body structures, to which they pass on the entraining signal.
Figure 12-14 diagrams how the pituitary gland works.
The SCN connects with pituitary endocrine neurons to control hormone release. A wide range of hormones circulates through the body to entrain many body tissues and organs.
Page 454The SCN also sends indirect messages to autonomic neurons in the spinal cord to inhibit the pineal gland from producing the hormone melatonin, which influences daily and seasonal biorhythms.
SCN cells themselves release hormones. Silver and colleagues (1996) used a transplantation technique in which encapsulated SCN cells were transplanted into hamsters that had received SCN lesions. Even though the transplanted cells did not make axonal connections, they restored many circadian behaviors, indicating that some SCN signals must be hormonal and travel through the bloodstream.
An illustration of the SCN’s widespread effects is its control of two hormones, melatonin and glucocorticoids. The SCN controls melatonin release from the pineal gland so that melatonin circulates during the dark phase of the circadian cycle. It also controls the release of glucocorticoids from the adrenal glands so that they circulate during the light phase of the circadian cycle.
Figure 2-30 diagrams the autonomic nervous system. Section 6-5 describes how glucocorticoids affect the body and brain.
Melatonin promotes sleep and influences the parasympathetic rest-
Pacemaking Circannual Rhythms
The suprachiasmatic nucleus controls not only daily rhythms but circannual rhythms as well. Russel Reiter (1980) first illustrated this form of pacemaking in hamsters. Hamsters are summertime (long-
During the dark phase of the day–
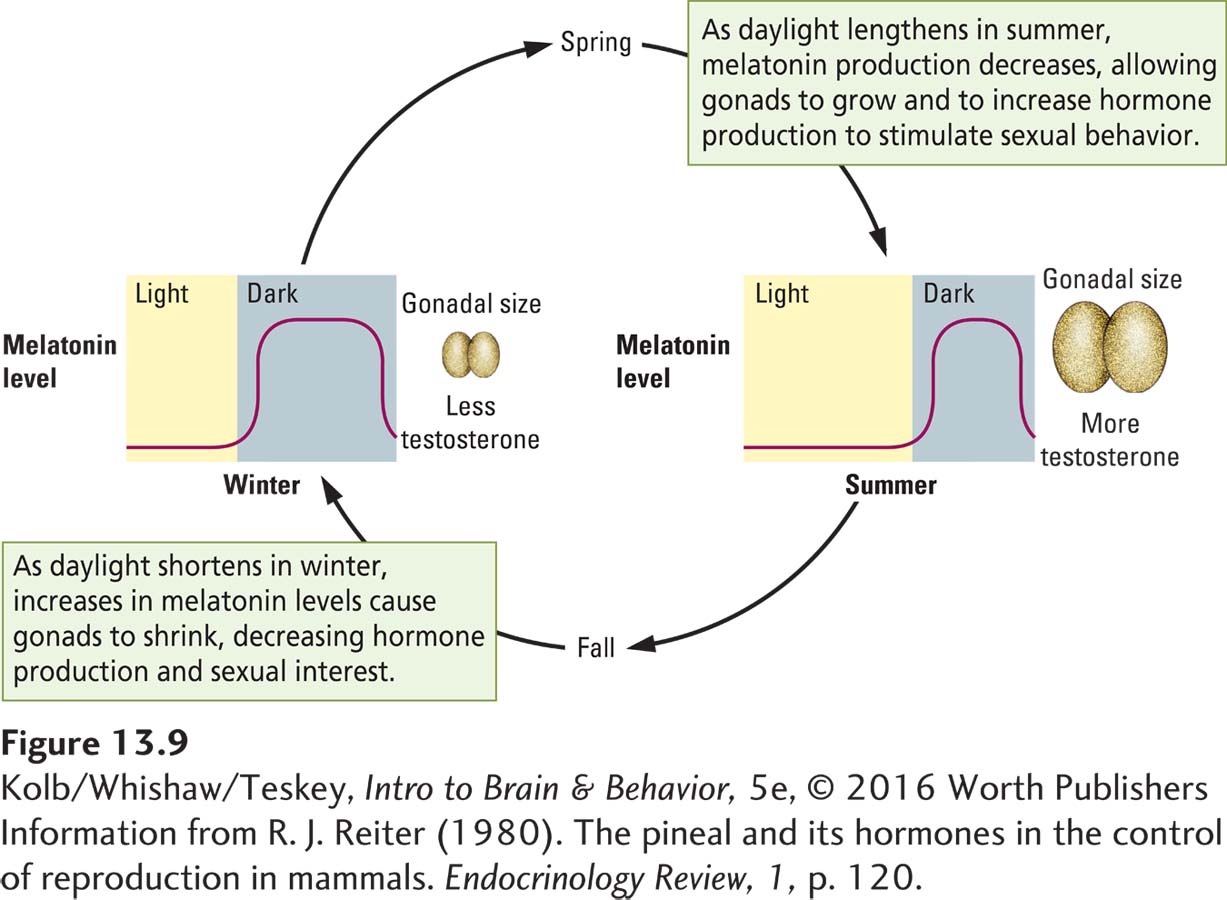
During the long daylight period of the circadian cycle the SCN inhibits melatonin secretion by the pineal gland. As the days become shorter, the melatonin inhibition period shortens and the release period lengthens. When the daylight period is shorter than 12 hours, melatonin release time becomes sufficient to inhibit the hamster’s gonads, and they shrink. Melatonin also influences the testes of short-
The SCN’s influence on circadian and circannual cycles is proposed to contribute to obesity in humans (Gangwisch, 2014). The idea is that long daylight hours involve fueling high-
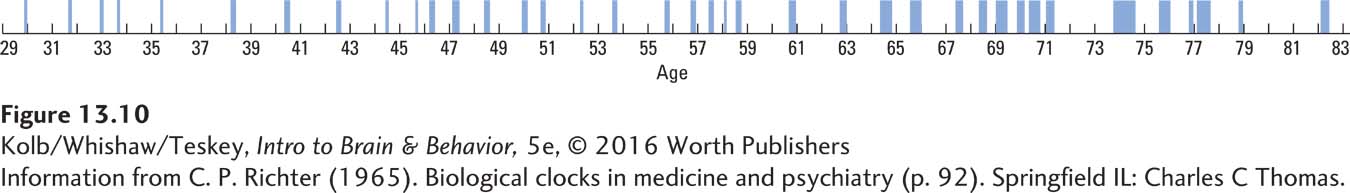
In his classic book Biological Clocks in Medicine and Psychiatry, Curt Richter (1965) hypothesized that many physical and behavioral disorders might be caused by shocks, either physical or environmental, that upset the timing of biological clocks. For example, the record of psychotic episodes of the English writer Mary Lamb illustrated in Figure 13-10 is one of many rhythmic records that Richter thought represented the action of an abnormally functioning biological clock.
Cognitive and Emotional Rhythms
Circadian rhythms are synchronized in many parts of the body. In a phasic manner they influence cognitive and emotional functions, including emotional experience, learning and retention, decision making, and motivation. The extent to which a process depends on the expenditure of metabolic energy dictates optimal times for its activity during the circadian cycle: time of day can serve as a good index for the time and place at which things should happen. Studies suggest that as much as 10 percent of the genome is under the epigenetic control of circadian rhythms.
Many cognitive events require changes in gene expression. Animals remember previous events better if they are tested at the time of the circadian period when the initial event occurred. Donald Cain and his colleagues (2008) used a conditional place response to test rats’ memories for a location at which they had previously received a small foot shock. The rats receive the noxious stimulation in a test box, then are returned to their home cage.
The following day groups of rats are tested at the time of training or a different time. Rats trained at time 1 and tested at time 1 show better retention as measured by the time they spent immobile (freezing) than rats trained at time 1 and tested at time 2, six hours later in the day. Similarly, rats trained at time 2 and tested at time 2 show better retention than rats trained at time 2 and tested at time 1, six hours earlier in the day. The results show that the circadian cycle puts a time stamp on the memory that allows it to be better recalled at the same time of the period that the initial event occurred.
Cognitive behaviors also synchronize with circadian periods. Animals have a variety of needs for food, water, and sleep, and it is adaptive for them to know when these resources become available. If food is available on a schedule, animals display anticipatory behaviors, becoming active as the feeding time arrives, for example. Other anticipatory events include salivation, intestinal activity, and sensations of hunger. Anticipatory activity might also include recalling events related to the timing of feeding and food location. Many cognitive activities can occur in the absence of the SCN, but it is adaptive for them to occur at the right time and place. SCN activity enables this. As animals age, their ability to associate appropriate activity with appropriate time declines, impairing their daily schedule, an effect that might in part account for poor scheduling and sleep in some older humans (Mulder et al., 2015).
Studies also find that the circadian period influences emotional behavior. A time of day effect may account for some of our emotional responses to daily events independent of the events themselves (Li et al., 2015). Nighttime fear is common, but is it the dark or the nighttime circadian rhythm that accounts for heightened emotion? By independently varying lighting conditions and test times during the circadian cycle, Li and colleagues found heightened emotional responses to stimuli at night independent of ambient lighting. Thus the cycle, not just darkness, contributes to emotional responding. Apparently at least two factors explain why horror movies watched at night are scarier: the movie’s content and the time of day effect.
13-2 REVIEW
Neural Basis of the Biological Clock
Before you continue, check your understanding.
Question 1
Biological rhythms are timed by internal biological clocks. The master clock is the ____________.
Question 2
Light cues entrain the suprachiasmatic nucleus to control daily rhythms via the ____________ tract, which receives information via ____________ cells.
Question 3
Pacemaking produced by the SCN is a product of its ____________ cells, which activate slave oscillators via both ____________ signals and ____________ connections.
Question 4
Why should studying for an exam and taking the exam occur at the same time of day?
Answers appear in the Self Test section of the book.