5-2 Varieties of Neurotransmitters and Receptors
Subsequent to Otto Loewi’s 1921 discovery that excitatory and inhibitory chemicals control heart rate, many researchers thought that the brain must work under much the same type of dual control. They reasoned that norepinephrine and acetylcholine were the transmitters through which excitatory and inhibitory brain cells worked. They did not imagine what we know today: the human brain employs a dazzling variety of neurotransmitters and receptors. The neurotransmitters operate in even more versatile ways: some may be excitatory at one location and inhibitory at another, for example, and two or more may team up in a single synapse so that one makes the other more potent. Moreover, each neurotransmitter may interact with several varieties of receptors, each with a somewhat different function.
In this section, you will learn how neurotransmitters are identified and how they fit within four broad categories on the basis of their chemical structure. The functional aspects of neurotransmitters interrelate and are intricate, with no simple one-
Four Criteria for Identifying Neurotransmitters
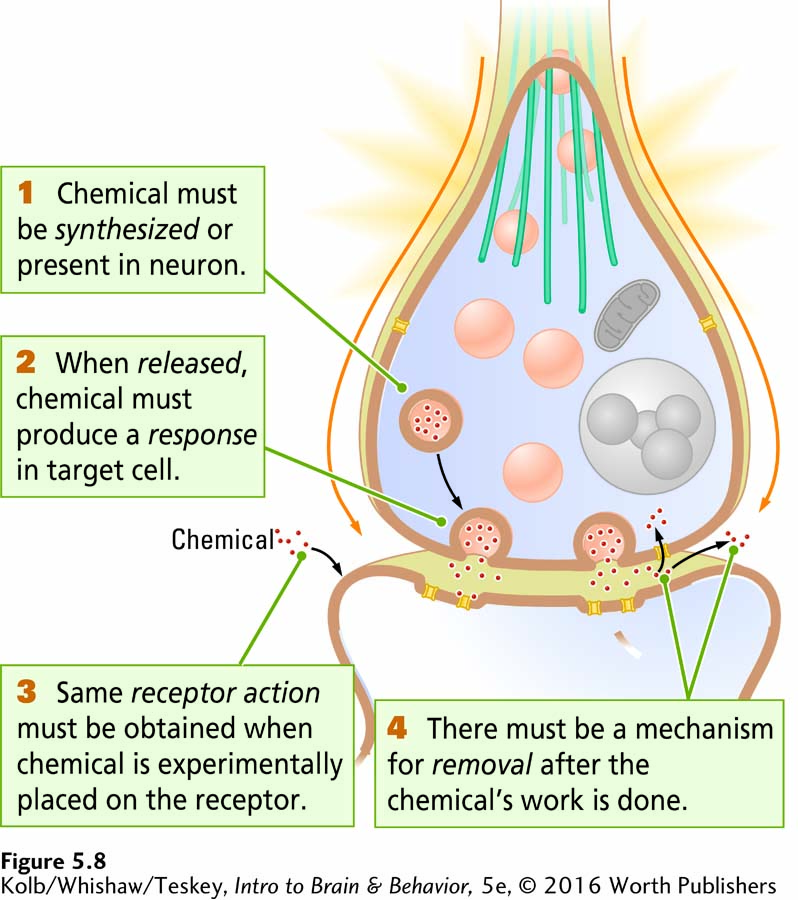
Among the many thousands of chemicals in the nervous system, which are neurotransmitters? Figure 5-8 presents four identifying criteria:
The chemical must be synthesized in the neuron or otherwise be present in it.
When the neuron is active, the chemical must be released and produce a response in some target.
Page 148The same response must be obtained when the chemical is experimentally placed on the target.
A mechanism must exist for removing the chemical from its site of action after its work is done.
These identifying criteria are fairly easy to apply for examining the somatic nervous system, especially at an accessible nerve–
Figure 4-6 illustrates the use of a glass microelectrode.
Researchers trying to identify new CNS neurotransmitters can use microelectrodes to stimulate and record from single neurons. A glass microelectrode is small enough to be placed on specific neuronal targets. It can be filled with a chemical of interest, and when a current is passed through the electrode, the chemical can be ejected into or onto the neuron to mimic neurotransmitter release onto the cell.
Many staining techniques can identify specific chemicals inside the cell. Methods have also been developed for preserving nervous system tissue in a saline bath while experimenters determine how the neurons in the tissue communicate. The use of such tissue slices simplifies the investigation by allowing the researcher to view a single neuron through a microscope while stimulating it or recording from it.
Acetylcholine was not only the first substance identified as a neurotransmitter but also the first substance identified as a CNS neurotransmitter. A logical argument that predicted its presence even before experimental proof was gathered greatly facilitated the process. All motor neuron axons leaving the spinal cord use acetylcholine as a transmitter. Each axon has an axon collateral within the spinal cord that synapses on a nearby CNS interneuron. The interneuron, in turn, synapses on the motor neuron’s cell body. This circular set of connections, called a Renshaw loop after the researcher who first described it, is shown in Figure 5-9.
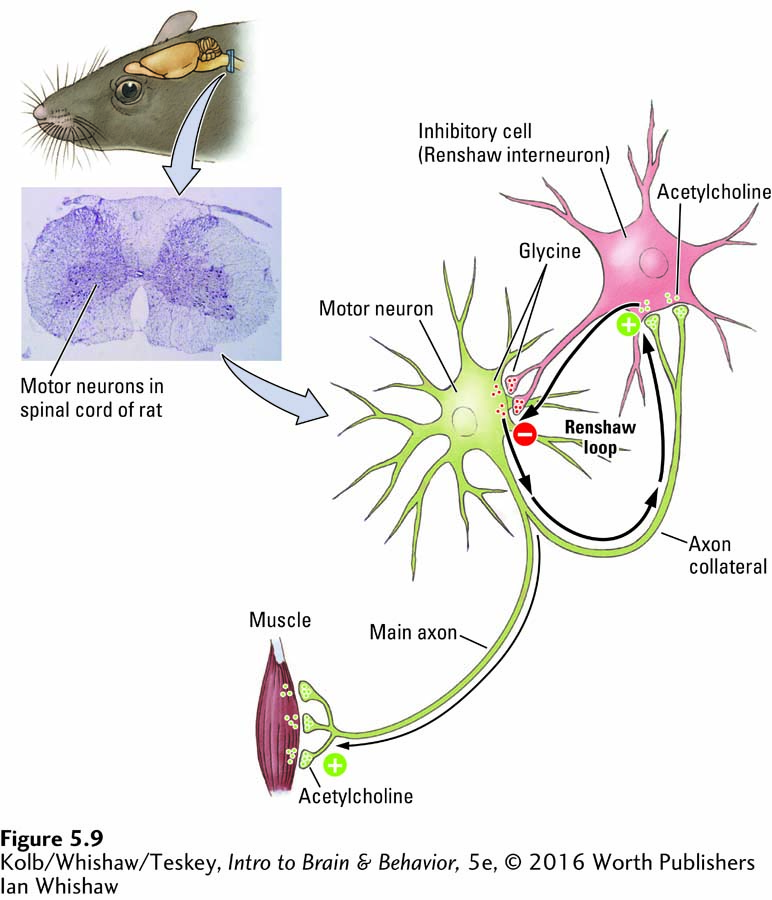
Because the main axon to the muscle releases acetylcholine, investigators suspected that its axon collateral also might release acetylcholine. For two terminals of the same axon to use different transmitters seemed unlikely. Knowing what chemical to look for made it easier to find and obtain the required evidence that ACh is in fact a neurotransmitter in both locations.
The loop made by the axon collateral and the interneuron in the spinal cord forms a feedback circuit that enables the motor neuron to inhibit itself from overexcitation, should it receive a great many excitatory inputs from other parts of the CNS. Follow the positive and negative signs in Figure 5-9 to see how the Renshaw loop works. If the loop is blocked, as can be done with the toxin strychnine, motor neurons become overactive, producing convulsions that can choke off respiration and so cause death.
The term neurotransmitter is used more broadly now than it was when researchers began to identify these chemicals. Today, the term applies to chemicals that
carry a message from the presynaptic membrane of one neuron to another by influencing postsynaptic membrane voltage.
change the structure of a synapse.
communicate by sending messages in the opposite direction. These retrograde (reverse-
direction) messages influence the release or reuptake of transmitters on the presynaptic side.
Four Classes of Neurotransmitters
We can impose some order on the diversity of neurotransmitters by classifying them into four groups based on their chemical composition: (1) small-
Small-
The first neurotransmitters identified are the quick-
Taking drugs orally is easy and comparatively safe, but not all drugs can traverse the digestive tract. Section 6-1 explains.
Because small-
Table 5-1 lists some of the best-
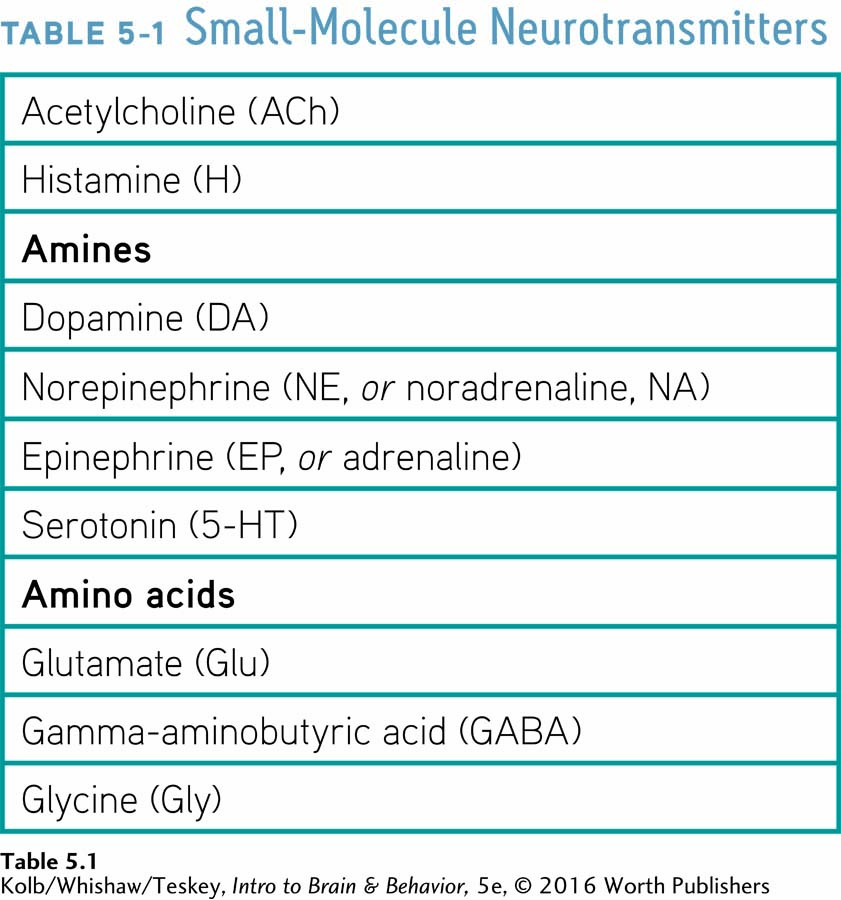
Among its many functions, which include control of arousal and of waking, the transmitter histamine (H) can cause the constriction of smooth muscles. When activated in allergic reactions, histamine contributes to asthma, a constriction of the airways. You are probably familiar with antihistamine drugs used to treat allergies.
ACETYLCHOLINE SYNTHESIS Acetylcholine is present at the junction of neurons and muscles, including the heart, as well as in the CNS. Figure 5-10 illustrates how ACh molecules are synthesized from choline and acetate by two enzymes, then broken down. Choline is among the breakdown products of fats in foods such as egg yolk, avocado, salmon, and olive oil; acetate is a compound found in acidic foods, such as vinegar and lemon juice.
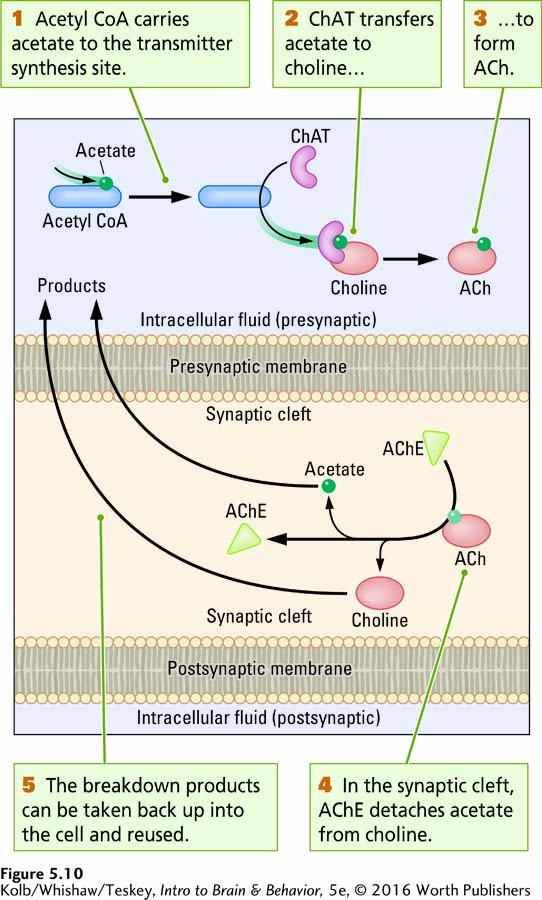
As depicted in Figure 5-10, inside the cell, acetyl coenzyme A (acetyl CoA) carries acetate to the synthesis site, and a second enzyme, choline acetyltransferase (ChAT), transfers the acetate to choline to synthesize acetylcholine. After ACh has been released into the synaptic cleft and diffuses to receptor sites on the postsynaptic membrane, a third enzyme, acetylcholinesterase (AChE), reverses the process, breaking down the transmitter by detaching acetate from choline. The breakdown products can then be taken back into the presynaptic terminal for reuse.
AMINE SYNTHESIS Some transmitters grouped together in Table 5-1 have common biochemical pathways to synthesis and so are related. You are familiar with the amines dopamine (DA), norepinephrine (NE), and epinephrine (EP). To review, DA loss figures in Parkinson disease, EP is the excitatory transmitter at the amphibian heart, and NE is the excitatory transmitter at the mammalian heart.
Figure 5-11 charts the biochemical sequence that synthesizes these amines in succession. The precursor chemical is tyrosine, an amino acid abundant in food. (Hard cheese and bananas are good sources.) The enzyme tyrosine hydroxylase (enzyme 1 in Figure 5-11) changes tyrosine into l-dopa, which other enzymes convert into dopamine, then norepinephrine, and, finally, epinephrine.
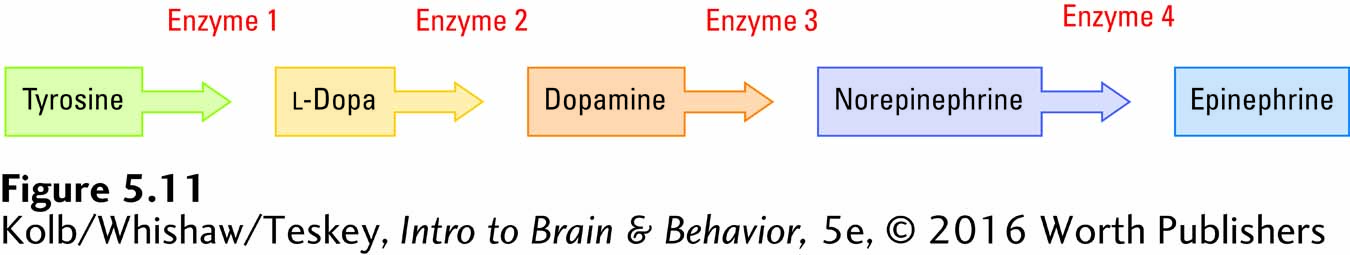
Interestingly, the supply of the enzyme tyrosine hydroxylase is limited. Consequently, so is the rate at which dopamine, norepinephrine, and epinephrine can be produced, regardless of how much tyrosine is present or ingested. This rate-
SEROTONIN SYNTHESIS The amine transmitter serotonin (5-
AMINO ACID SYNTHESIS Two amino acid transmitters, glutamate (Glu) and gamma-
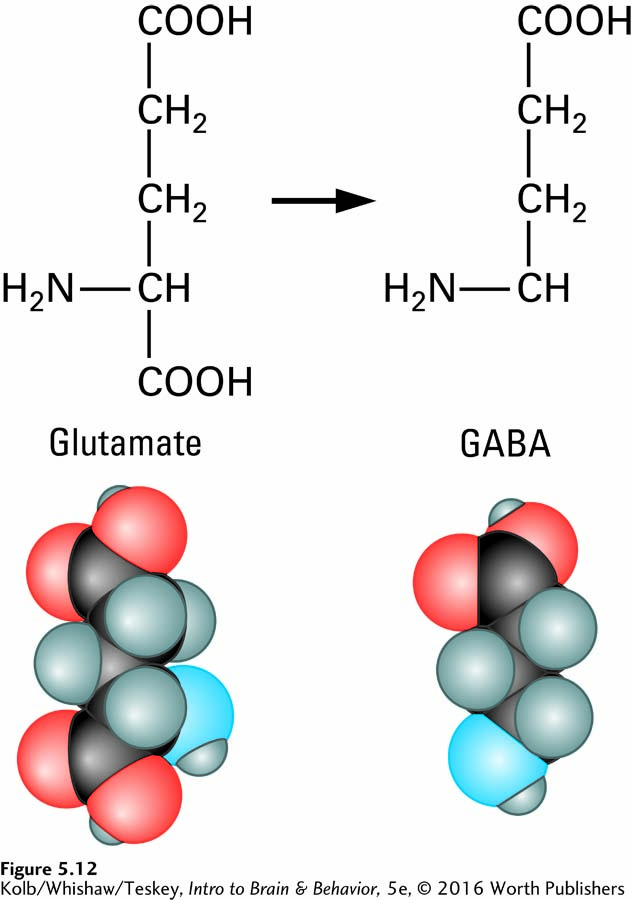
In the forebrain and cerebellum, glutamate is the main excitatory transmitter and GABA is the main inhibitory transmitter. Thus, glutamate is a neurotransmitter in excitatory synapses, and GABA is a neurotransmitter in inhibitory synapses. So a synapse’s appearance provides information about the neurotransmitter and its function (review Figure 5-9).
Peptide Transmitters
More than 50 short amino acid chains of various lengths (fewer than 100) form the families of peptide transmitters, or neuropeptides, listed in Table 5-2. Synthesized through the translation of mRNA from instructions contained in the neuron’s DNA, neuropeptides are multifunctional chains of amino acids that act as neurotransmitters.
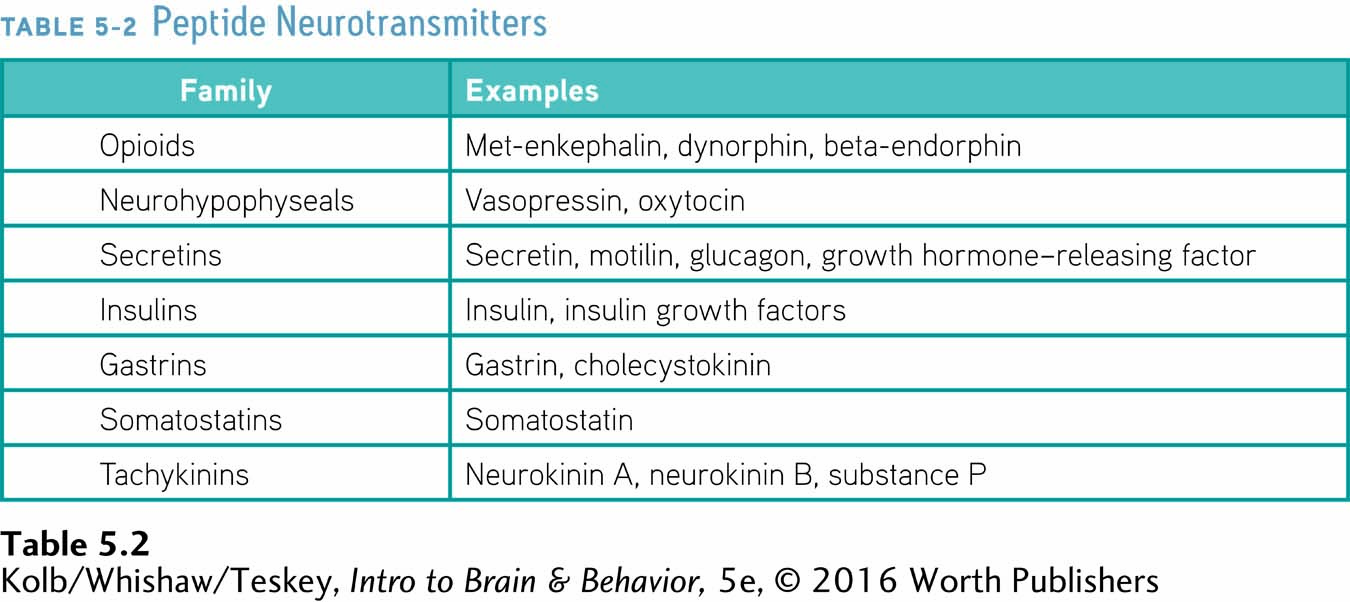
Figure 3-15 diagrams peptide bonding and Figure 3-17, protein export.
In some neurons, peptide transmitters are made in the axon terminal, but most are assembled on the neuron’s ribosomes, packaged in a membrane by Golgi bodies, and transported by the microtubules to the axon terminals. The entire process of neuropeptide synthesis and transport is relatively slow compared with the nearly ready-
Neuropeptides, however, perform an enormous range of functions in the nervous system, as might be expected from their large numbers. They act as hormones that respond to stress, enable a mother to bond with her infant, regulate eating and drinking and pleasure and pain, and probably contribute to learning.
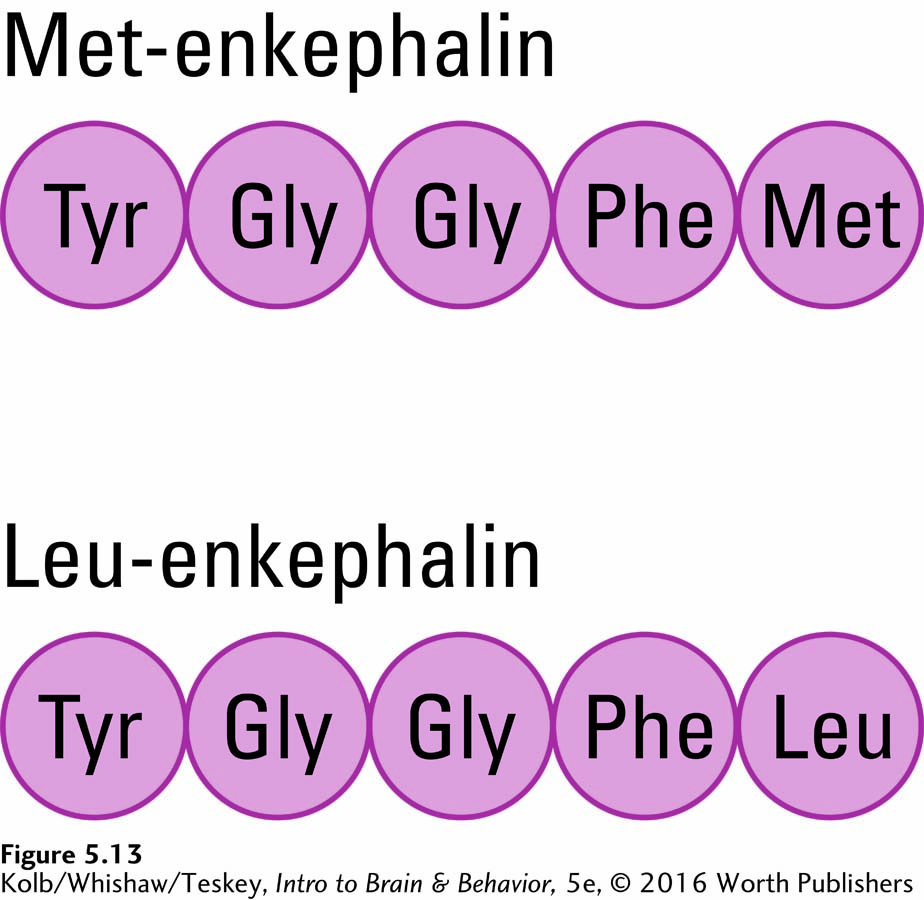
Opium and related synthetic chemicals such as morphine, long known both to produce euphoria and to reduce pain, appear to mimic the actions of endogenous brain opioid neuropeptides: enkephalins, dynorphins, and endorphins. (The term enkephalin derives from the phrase in the cephalon, meaning in the brain or head, whereas the term endorphin is a shortened form of endogenous morphine.)
A part of the amino acid chain in each of these naturally occurring opioid peptides is structurally similar to the others, as illustrated for two of them in Figure 5-13. Presumably, opium mimics this part of the chain. The discovery of naturally occurring opium-
Some CNS peptides take part in specific periodic behaviors, each month or each year perhaps. For instance, in female deer, neuropeptide transmitters act as hormones (luteinizing hormone) that prepare her for the fall mating season. Come winter, a different set of biochemicals facilitates the developing deer fetus. When the mother gives birth in the spring, yet another set of highly specific neuropeptide hormones—
Sections 12-4 and 12-5 explain hormonal influence over human emotional and motivated behavior.
The same neuropeptides serve similar specific hormonal functions in humans. Others, such as neuropeptide growth hormones, perform far more general functions in regulating growth. Unlike small-
Lipid Transmitters
Predominant among the lipid neurotransmitters are the endocannabinoids (endogenous cannabinoids), a class of lipid neurotransmitters synthesized at the postsynaptic membrane to act on receptors at the presynaptic membrane. The endocannabinoids include anandamide and 2-
5-3
Awakening with l-Dopa
He was started on l-dopa in March 1969. The dose was slowly raised to 4.0 mg a day over a period of three weeks without apparently producing any effect. I first discovered that Mr. E. was responding to l-dopa by accident, chancing to go past his room at an unaccustomed time and hearing regular footsteps inside the room. I went in and found Mr. E., who had been chair bound since 1966, walking up and down his room, swinging his arms with considerable vigor, and showing erectness of posture and a brightness of expression completely new to him. When I asked him about the effect, he said with some embarrassment: “Yes! I felt the l-dopa beginning to work three days ago—
In this case history, neurologist Oliver Sacks describes administering l-dopa to a patient who had acquired parkinsonism as an aftereffect of severe influenza in the 1920s. The relation between the influenza and the parkinsonian symptoms suggests that the flu virus had entered the brain and selectively attacked dopamine neurons in the substantia nigra. By increasing the amount of DA in remaining synapses, l-dopa relieved the patient’s symptoms.
Two separate groups of investigators independently gave l-dopa to Parkinson patients beginning in 1961 (Birkmayer & Hornykiewicz, 1961; Barbeau et al., 1961). Both research teams knew that the chemical is catalyzed into dopamine at DA synapses (see Figure 5-11). The l-dopa turned out to reduce the patients’ muscular rigidity.
This work was the first demonstration that a neurological condition can be relieved by a drug that aids in increasing the amount of a neurotransmitter. l-Dopa has since become a standard treatment for Parkinson disease. Its effects have been improved by the administration of drugs that prevent l-dopa from being converted to dopamine in the body before it passes through the blood–
l-Dopa is not a cure. Parkinson disease still progresses during treatment, and as more and more dopamine synapses are lost, the treatment becomes less and less effective. Eventually, l-dopa begins to produce dyskinesias—involuntary, unwanted movements, such as ballistic (throwinglike) or choreic (dancelike) movements. When these side effects eventually become severe, the treatment must be discontinued.

Fatty acid molecules that contribute to forming the cell membrane likewise are hydrophobic. See Figure 3-11.
Because endocannabinoids are lipophilic (fat-
The CB1 receptor is the target of all cannabinoids, whether generated by the body (endocannabinoids), from plants (phytocannabinoids), or synthetically. CB1 receptors are found at both glutamate and GABA synapses, and so cannabinoids act as neuromodulators to inhibit release of glutamate and GABA. Cannabinoids thus dampen both neuronal excitation and inhibition.
Cannabis is among the psychotropic drugs discussed in Section 6-2.
Phytocannabinoids are obtained from the hemp plants Cannabis sativa and Cannabis indica. These plants have been used medically and recreationally for thousands of years, but only recently was an extract from cannabis synthesized. Early in the last century, many constituents of cannabis, including cannabidiol and tetrahydrocannabinol (THC), were isolated and their chemical structure determined. In 1964, Yehiel Gaoni and Raphael Mechoulam reported the structure of the THC molecule, the main psychoactive constituent in cannabis. Next, investigators determined how THC is metabolized. (The process is quite slow, which explains why THC can be detected in urine for weeks after cannabis use.)
Research on the physiological and psychological effects of THC in animals and people, which began after its isolation and purification, is ongoing. Twenty-
Gaseous Transmitters
The gases nitric oxide (NO), carbon monoxide (CO), and hydrogen sulfide (H2S) further expand the biochemical strategies that transmitter substances display. As water-
All three gaseous transmitters serve as chemical messengers in many parts of the body. NO and H2S control intestinal wall muscles and dilate blood vessels in active brain regions, allowing these regions to receive more blood. Because NO and H2S also dilate blood vessels in the sexual organs, both are active in producing penile erections. Drugs used to treat erectile dysfunction in men, such as Viagra and Cialis, act by enhancing the chemical pathways influenced by NO. NO does not of itself produce sexual arousal.
Varieties of Receptors
Each of the two general classes of receptor proteins produces a different effect: one directly changes the postsynaptic membrane’s electrical potential, and the other induces cellular change indirectly. A dazzling array of receptor subtypes allows for subtle differences in receptor function.
Two Classes of Receptors
When a neurotransmitter is released from any of the wide varieties of synapses onto a wide variety of targets, as illustrated in Figure 5-6, it crosses the synaptic cleft and binds to a receptor. What happens next depends on the receptor type.
Structurally, ionotropic receptors resemble voltage-
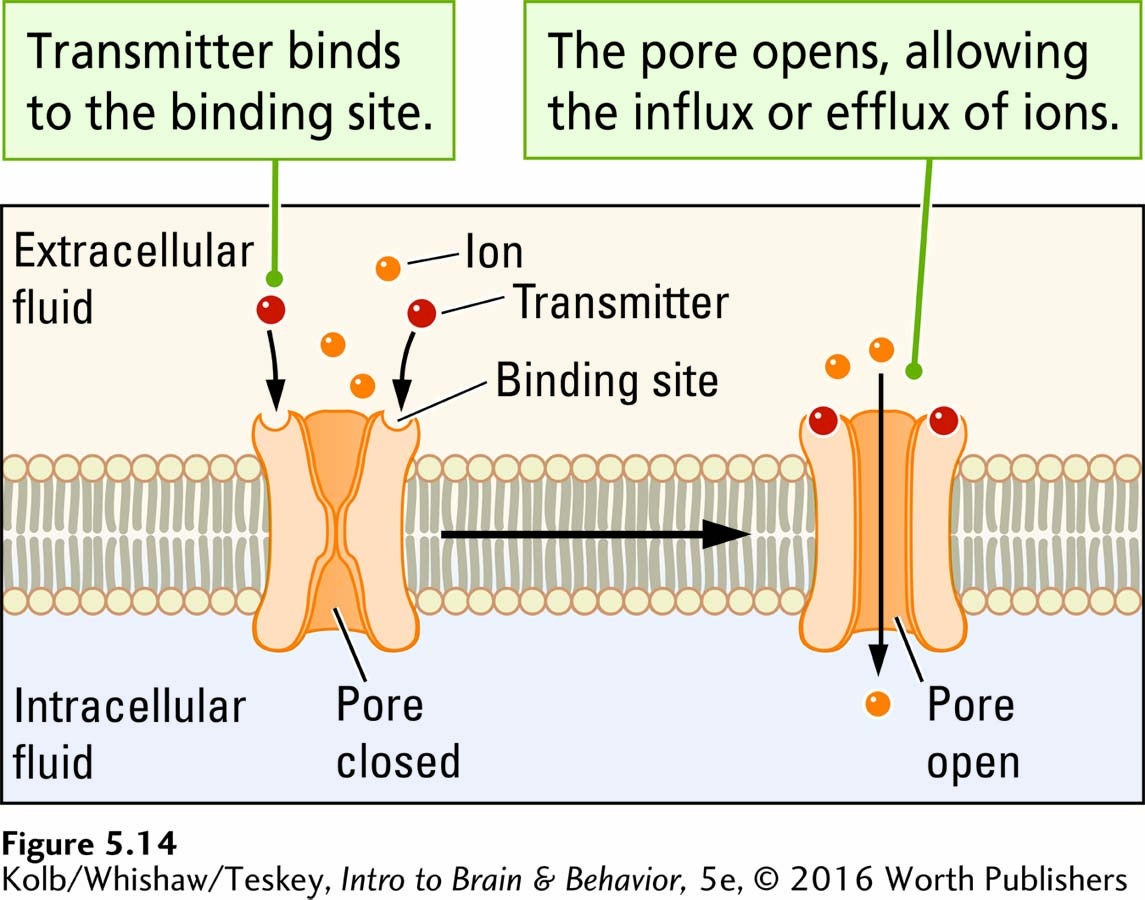
Ionotropic receptors allow the ions, such as Na+, K+, and Ca2+, to move across a membrane (the suffix -tropic means moving toward). As Figure 5-14 illustrates, an ionotropic receptor has two parts: (1) a binding site for a neurotransmitter and (2) a pore, or channel. When the neurotransmitter attaches to the binding site, the receptor quickly changes shape, either opening the pore and allowing ions to flow through it or closing the pore and blocking the ion flow. Thus, ionotropic receptors bring about rapid changes in membrane voltage and are usually excitatory: they trigger an action potential.
In contrast, a metabotropic receptor has a binding site for a neurotransmitter but lacks its own pore through which ions can flow. Through a series of steps, activated metabotropic receptors indirectly produce changes in nearby membrane-
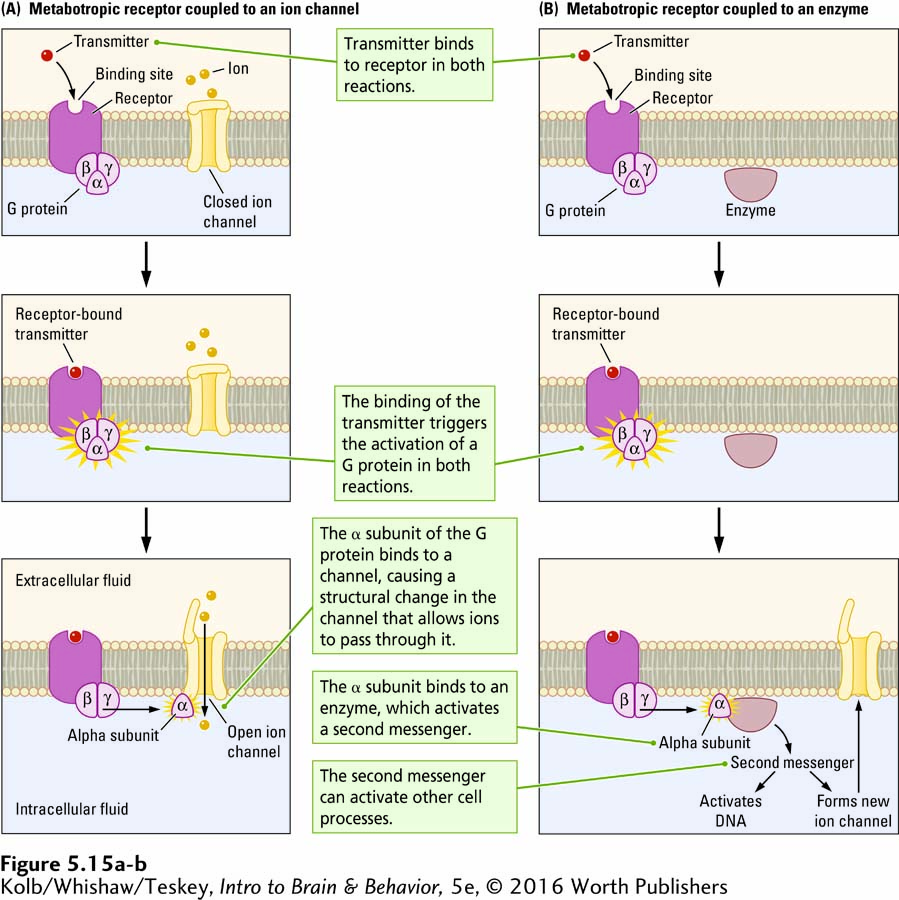
A G protein consists of three subunits: alpha, beta, and gamma. (A subunit is a protein that assembles with other proteins.) The alpha subunit detaches when a neurotransmitter binds to the G protein’s associated metabotropic receptor. The detached alpha subunit can then bind to other proteins within the cell’s membrane or its intracellular fluid. If the alpha subunit binds to a nearby ion channel in the membrane, as shown at the bottom of Figure 5-15A, the channel structure changes, modifying the flow of ions through it. If the channel is open, the alpha subunit may close it or, if closed, it may open. Changes in the channel and the ion flow across the membrane influence the membrane’s electrical potential.
The binding of a neurotransmitter to a metabotropic receptor can also trigger more complicated cellular reactions, summarized in Figure 5-15B. All these reactions begin when the detached alpha subunit binds to an enzyme. The enzyme in turn activates a second messenger (the neurotransmitter being the first messenger) that carries instructions to other cellular structures. As illustrated at the bottom of Figure 5-15B, the second messenger can
bind to a membrane-
bound channel, causing the channel to change its structure and thus alter ion flow through the membrane. initiate a reaction that incorporates intracellular (within the cell) protein molecules into the cell membrane, resulting, for example, in the formation of new ion channels.
bind to sites on the cell’s DNA to initiate or cease the production of specific proteins.
Metabotropic receptors also allow for the possibility that a single neurotransmitter’s binding to a receptor can activate an escalating sequence of events called an amplification cascade. The cascade effect is that many downstream proteins (second messengers or channels or both) are activated or deactivated. Ionotropic receptors do not have such a widespread amplifying effect.
Recall that acetylcholine has an excitatory effect on skeletal muscles. Here it activates an ionotropic receptor. Conversely, acetylcholine has an inhibitory effect on the heart rate. Here it activates a metabotropic receptor. Further, each transmitter may bind with several different kinds of ionotropic or metabotropic receptors. Elsewhere in the nervous system, for example, ACh may activate a wide variety of either receptor type.
Receptor Subtypes
While there are two general classes of receptors, ionotropic and metabotropic, each neurotransmitter may interact with a number of receptor subtypes specific to that neurotransmitter. Serotonin (5-
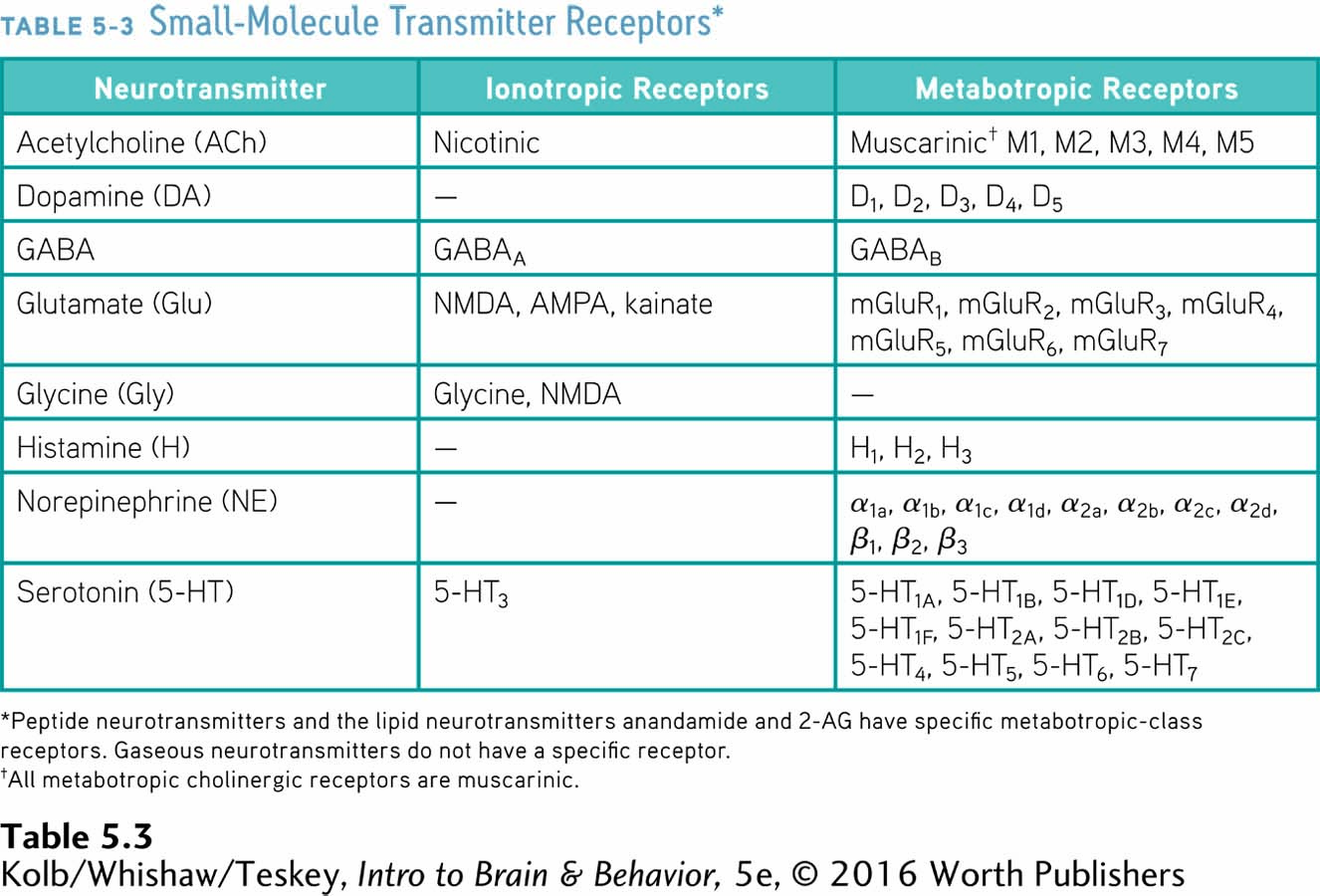
Figure 14-18 diagrams how glutamate and the NMDA receptor function in associative learning.
How is this variety achieved? Alternative forms of each subunit can assemble in unique combinations to make a functional receptor. For instance, the functional NMDA receptor, an ionotropic receptor for glutamate, is always composed of 4 subunits, but a total of 12 distinct subunits are available to come together in various combinations to form the functional receptor.
Why does the brain contain so many receptor subtypes for each neurotransmitter? The answer seems to be that each subtype has slightly different properties, which confer different activities. These activities can include the presence or absence of binding sites for other molecules, how long a channel remains open or closed, and the ability to interact with intracellular signaling molecules.
It should not be surprising that a brain such as ours, with its incredible complexity, is built upon a vast array of units, including copious neurotransmitter types and even more copious receptor types. All this, and more, allows the human brain to function successfully.
5-2 REVIEW
Varieties of Neurotransmitters and Receptors
Before you continue, check your understanding.
Question 1
Neurotransmitters are identified using four experimental criteria: ______, ______, ______, and ______.
Question 2
The four broad classes of chemically related neurotransmitters are ______, ______, ______, and ______.
Question 3
Acetylcholine is composed of ______ and ______. After release into the synaptic cleft, ACh is broken down by ______, and the products can be recycled.
Question 4
Endocannabinoids are ______ neurotransmitters, made on demand and released from the ______ membrane.
Question 5
Contrast the major characteristics of ionotropic and metabotropic receptors.
Answers appear in the Self Test section of the book.