12-5 Control of Regulatory and Nonregulatory Behavior
The two distinctly different types of motivated behaviors described in Section 12-4 are regulatory behaviors, which maintain vital body system balance, or homeostasis; and nonregulatory behaviors, those not controlled by a homeostatic mechanism—
Controlling Eating
Feeding behavior entails far more than sustenance alone. We must eat and drink to live, but we also derive great pleasure from these acts. For many people, eating is a focus of daily life, if not for survival, for its centrality to social activities, from get-
Control over eating is a source of frustration and even grief for many people in the developed world. In 2000, the World Health Organization identified obesity, the excessive accumulation of body fat, as a worldwide epidemic. The United States is a case in point. From 1990 to 2010, the proportion of overweight people increased from about 50 percent to 65 percent of the population. The proportion of people considered obese increased from about 12 percent in 1990 to 33 percent in 2014.
The increasing numbers of overweight and obese children and adults persist despite a substantial decrease in fat intake in American diets. What behaviors might cause persistent weight gain? One key to understanding weight gain in the developed world is evolutionary. Even 40 years ago, much of our food was only seasonally available. In a world with uncertain food availability, it makes sense to store excess body calories in the form of fat to be used later when food is scarce. Down through history and in many cultures today, plumpness was and is desirable as a standard of beauty and a sign of health and wealth.
In postindustrial societies, where food is continuously and easily available, overweight may not be the healthiest condition. People eat as though food will be scarce and fail to burn off the extra calories by exercising, and the result is apparent. About half of the U.S. population has dieted at some point in their life. At any given time, at least 25 percent report that they are currently on a diet. For a comparison of how some well-
Most Americans are overweight despite living in a culture obsessed with slimness. The human control system for feeding has multiple neurobiological inputs, including cognitive factors such as thinking about food and the association between environmental cues (e.g., watching television or studying) and the act of eating. The constant pairing of such cues with eating can result in the cues alone becoming a motivation—
Eating disorders entail being either overweight or underweight. Anorexia nervosa is an eating disorder with a huge cognitive component: self-
The neurobiological control of feeding behavior in humans is not as simple as it is in the fly described in Section 12-3. The multiple inputs to the human control system for feeding come from three major sources: the cognitive factors already introduced, the hypothalamus, and the digestive system.
Digestive System and Control of Eating
Figure 2-31 diagrams the inner workings of the ENS and Section 5-3 the main neurotransmitters it employs.
As illustrated in Figure 12-23, the digestive tract begins in the mouth and ends at the anus. Digestion is controlled by the enteric nervous system. As food travels through the tract, the digestive system extracts three types of nutrients: lipids (fats), amino acids (the building blocks of proteins), and glucose (sugar). Each nutrient is a specialized energy reserve. Because we require varying amounts of these reserves depending on what we are doing, the body has detector cells to keep track of the level of each nutrient in the bloodstream.
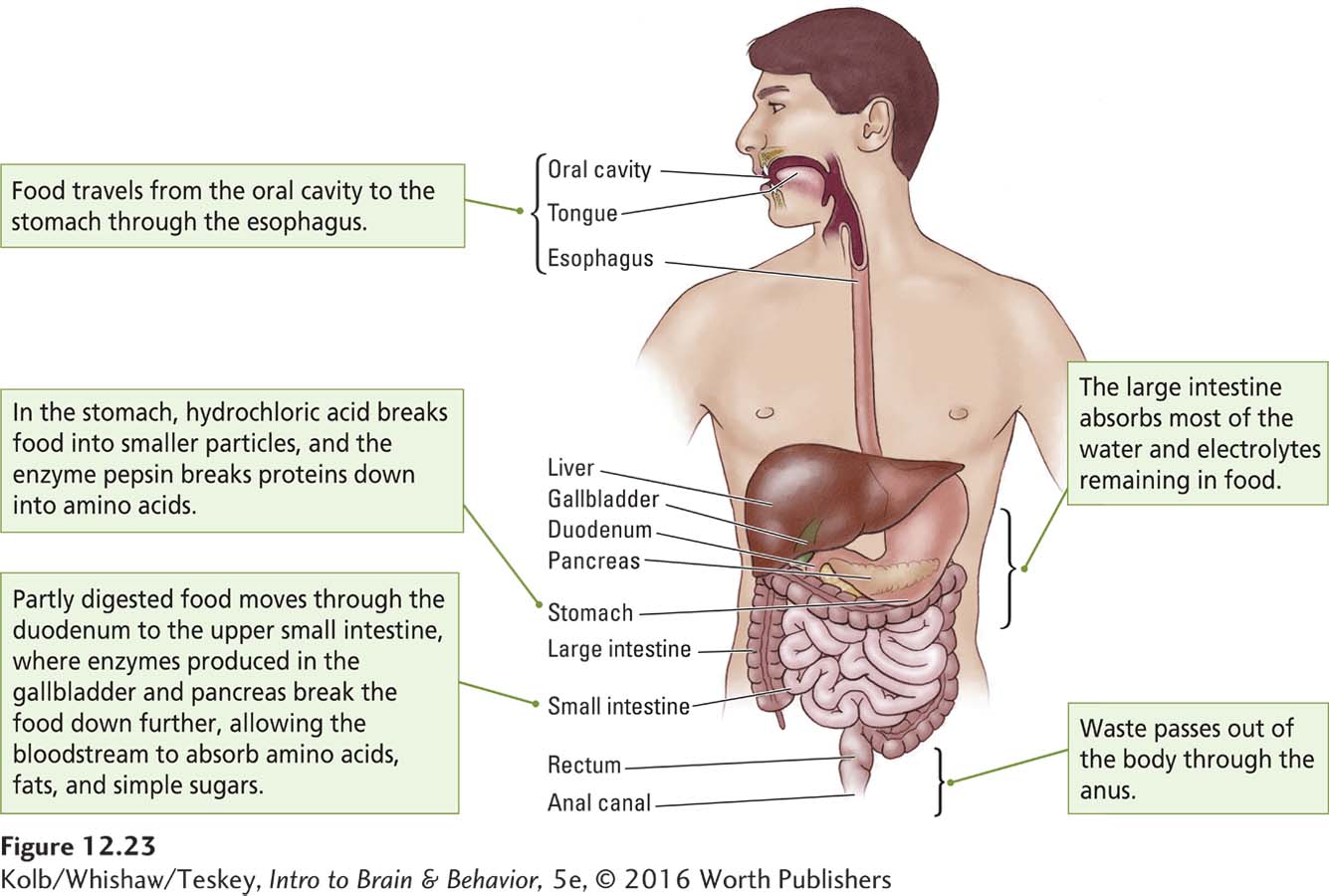
Glucose is the body’s primary fuel and virtually the only energy source for the brain. Because the brain requires glucose even when the digestive tract is empty, the liver acts as a short-
Thus the digestive system functions mainly to break down food, and the body needs to be apprised of how this breakdown is proceeding. Feedback mechanisms provide such information. When food reaches the intestines, it interacts with receptors in the ENS to trigger the release of at least 10 different peptide hormones, including cholecystokinin (CCK), glucagonlike peptide 1 (GLP-
12-4
Weight Loss Strategies
Among the wide range of diets and weight loss strategies on the market, none has stopped the obesity epidemic facing the developed world. Diets range widely in their recommended allowed proportions and types of fats, carbohydrates, and proteins.
A study by Iris Shai and colleagues (2008) compared a low-
Figure A shows that all three diets led to weight loss. The low-
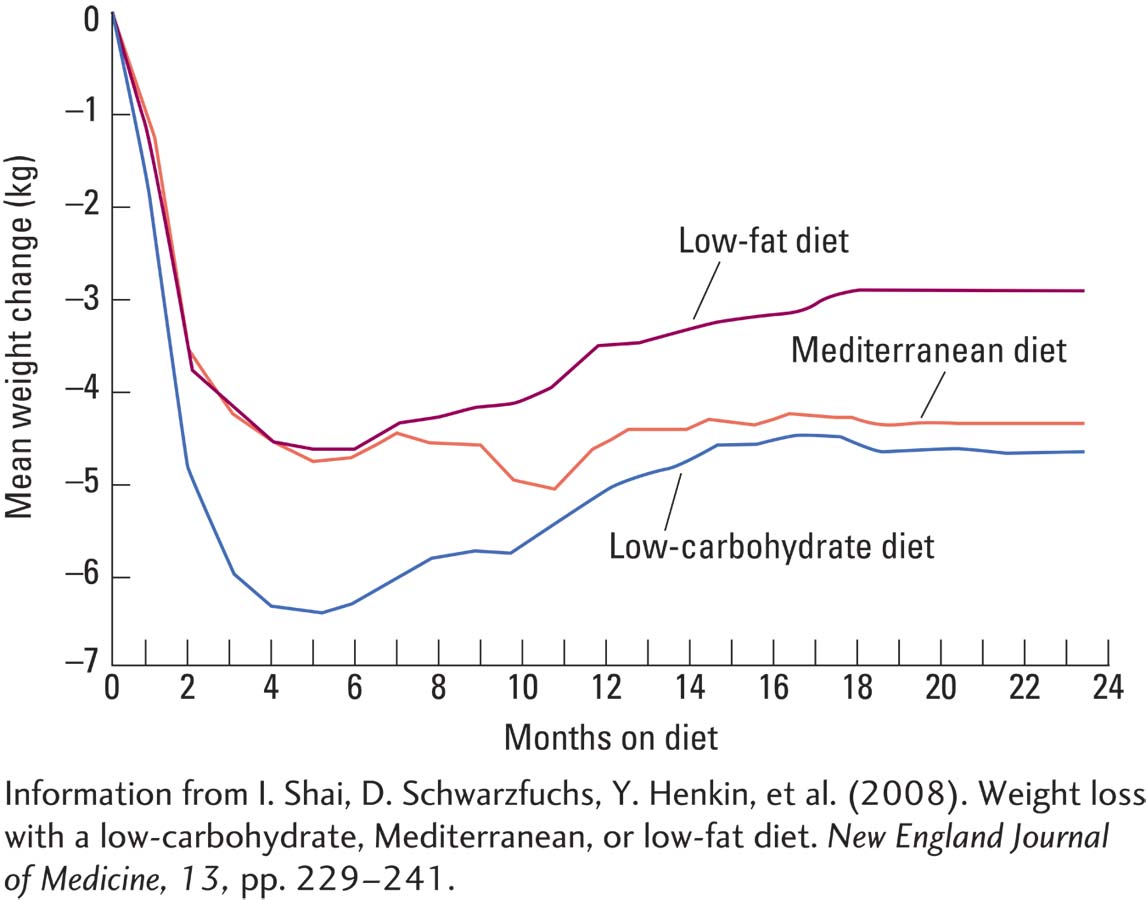
The low-
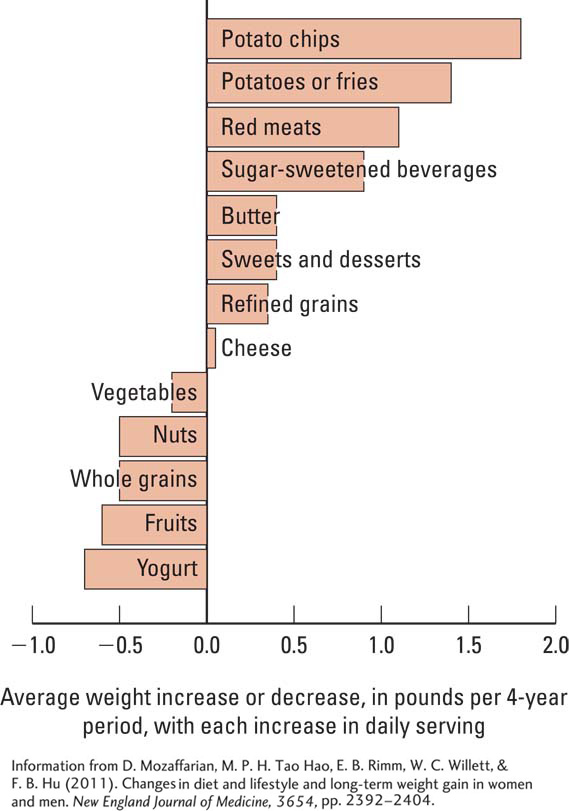
The question of which foods are most likely to lead to weight gain was studied in a 20-
Shown graphically in Figure B, weight gain was most strongly related to the intake of potato chips, potatoes, sugar-
Teresa Fung and her colleagues (2010) conducted a prospective study of mortality over a 26-
Shai and her colleagues concluded that health care professionals might suggest more than one dietary approach based on individual preferences and metabolic needs, as long as the effort is sustained. At present, the only certain formula for weight loss appears to include (1) a permanent switch to a diet reduced in calories and fat and (2) increased ingestion of high-
Hypothalamus and Control of Eating
Section 6-5 reviews the general categories of hormones and how they work.
Feeding behavior is influenced by hormones, including insulin, growth hormone, and sex steroids, that stimulate and inhibit feeding and aid in converting nutrients into fat and fat into glucose. The hypothalamus, which controls hormone systems, is the key brain structure in feeding as well as in satiety.
Investigation into how the hypothalamus controls feeding began in the early 1950s, when researchers discovered that damage to the lateral hypothalamus in rats caused the animals to stop eating, a symptom known as aphagia (in Greek, phagein means to eat). In contrast, damage to the ventromedial hypothalamus (VMH) caused the animals to overeat—
The researchers also found that electrical stimulation of the lateral hypothalamus elicits feeding, whereas stimulation of the ventromedial hypothalamus inhibits feeding. The opposing effects of injury and stimulation to these two regions led to the idea that the lateral hypothalamus signals turn eating on, whereas the VMH signals turn eating off. This model quickly proved too simple.
Not only does the lateral hypothalamus contain cell bodies; fiber bundles also pass through it. Damage to either the cell bodies or the fibers can produce aphagia. Similarly, damage to fibers passing through the VMH often causes injury as well to the paraventricular nucleus of the hypothalamus (review Figure 12-12A). But the role of the hypothalamus in controlling feeding involves more than the activities of its lateral and ventromedial structures alone.
In fact, another hypothalamic region, the arcuate nucleus, contains two major classes of neurons, one that initiates eating (e.g., neurons expressing genes for neuropeptide Y) and one that reduces eating behavior, the principal transmitter being a-
EXPERIMENT
Question: Does the hypothalamus play a role in eating?
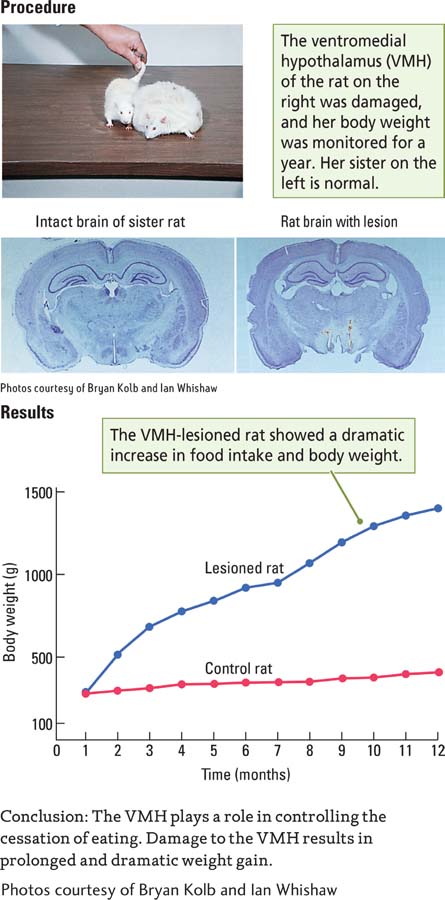
The summed activity of all such hypothalamic neurons constitutes a complex homeostat that controls feeding. Figure 12-24 shows that this homeostat receives inputs from three sources: the enteric nervous system (such as information about blood glucose levels), hormone systems (such as information about the level of appetite-
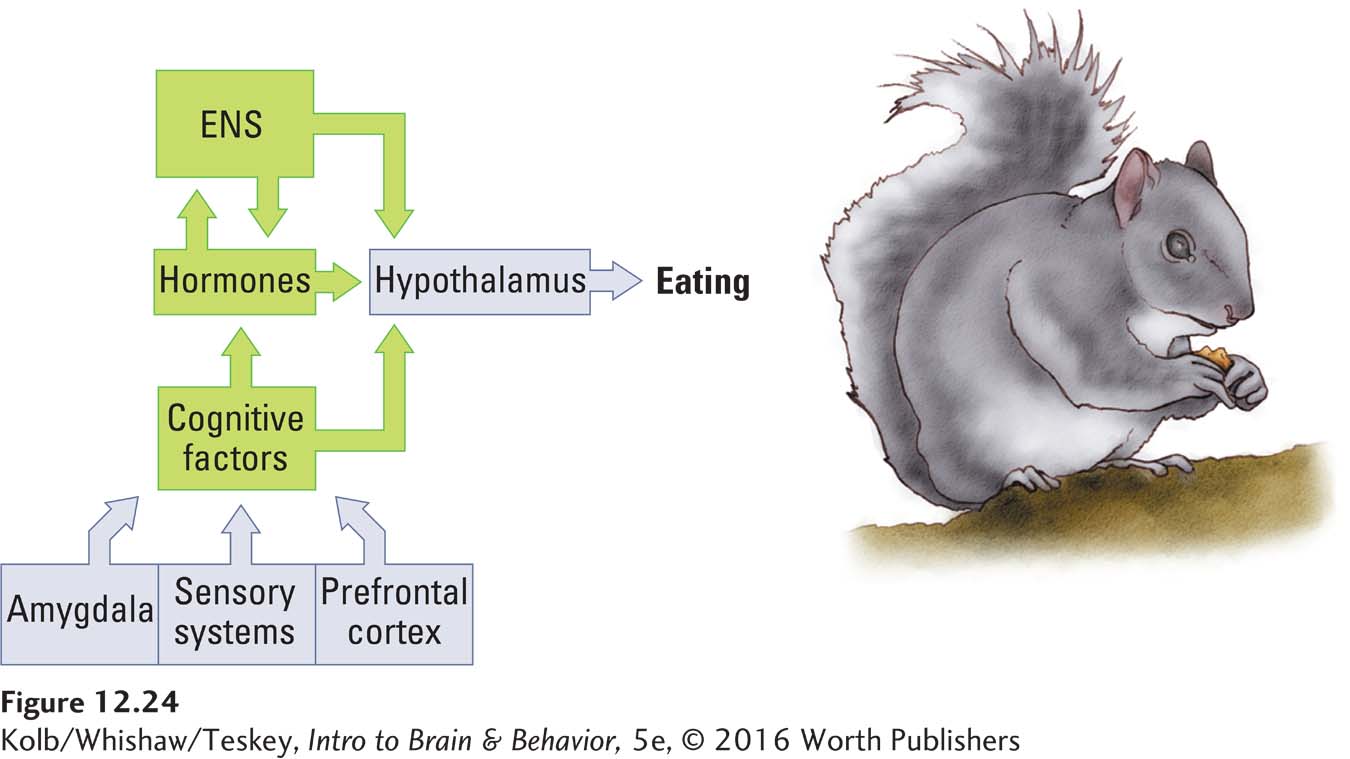
Cognitive Control of Eating
Pleasure and its absence are cognitive factors in controlling eating. Just thinking about a favorite food can make many of us feel hungry. The cognitive aspect to feeding includes not only images of food that we pull from memory but also external sensations, especially food-
Neural control of the cognitive factors important for controlling eating in humans probably originates in multiple brain regions. Two structures are clearly important: the amygdala and the orbital prefrontal cortex. Damage to the amygdala alters food preferences and abolishes taste aversion learning. These effects are probably related to the amygdala’s efferent connections to the hypothalamus.
The amygdala’s role in regulating species-
An additional cognitive factor in control of eating is the pleasure we derive from it, especially from eating foods with certain tastes. Think chocolate. What pleasure is and how the brain produces it are discussed in Section 12-6 in the context of reward.
Randy Seeley and Stephen Woods (2003) noted that in spite of contemporary problems with weight gain, adult mammals do a masterful job of matching their caloric intake to caloric expenditure. Consider that a typical man eats 900,000 calories per year. To gain just one extra pound requires him to eat 4000 calories more than he burned in that year. This increase amounts to only 11 calories per day, equivalent to a single potato chip. But people rarely eat just one chip. As Figure B in Clinical Focus 12-4 illustrates, potato chips top the list of major food sources linked to weight gain over time.
Controlling Drinking
Dissolved in the water that is 70 percent of the human body are chemicals that participate in the hundreds of reactions necessary to bodily functions. Essential homeostatic mechanisms control water levels (and hence chemical concentrations) within rather narrow limits. The rate of a chemical reaction is partly determined by the concentration of the participating chemicals.
As with eating, we drink for many reasons. We consume some beverages, such as coffee, wine, beer, and juice, for an energy boost or to relax, as part of social activities, or just because they taste good. We drink water for its health benefits, to help wash down a meal or to intensify the flavor of dry foods. On a hot day, we drink water because we are thirsty, presumably because we become dehydrated through sweating and evaporation.
These examples illustrate the two kinds of thirst. Osmotic thirst results from increased concentrations of dissolved chemicals, known as solutes, in the body fluids. Hypovolemic thirst results from a loss of overall fluid volume from the body.
Osmotic Thirst
Solutes found inside and outside cells are ideally concentrated for the body’s chemical reactions. Maintaining this concentration requires a kind of homeostat, much like the mechanism that controls body temperature. Deviations from the ideal solute concentration activate systems to reestablish it.
Turning to sugar-
When we eat salty foods, such as potato chips, the salt (NaCl) spreads through the blood and enters the extracellular fluid between our cells. This shifts the solute concentration away from the ideal. Receptors in the hypothalamus along the third ventricle detect the altered solute concentration and relay the message too salty to various hypothalamic areas that in turn stimulate us to drink. Other messages are sent to the kidneys to reduce water excretion.
Water Intoxication
Eating too much leads to obesity. What happens when we drink too much water? Our kidneys are efficient at processing water, but if we drink a large volume all at once, the kidneys cannot keep up.
The result is a condition called water intoxication. Body tissues swell with the excess fluid, essentially drowning the cells in freshwater. At the same time, the relative concentration of sodium drops, leading to an electrolyte imbalance.
Water intoxication can produce widely ranging symptoms, from irregular heartbeat to headache. In severe cases, people may act as though they are drunk. The most likely way for an adult to develop water intoxication is to sweat heavily, by running a marathon in hot weather, for example, then drink too much water without added electrolytes.
Hypovolemic Thirst
Unlike osmotic thirst, hypovolemic thirst arises when the total volume of body fluids declines, motivating us to drink more and replenish them. In contrast with osmotic thirst, however, hypovolemic thirst encourages us to choose something other than water, because water would dilute the solute concentration in the blood. Rather, we prefer to drink flavored beverages that contain salts and other nutrients.
Hypovolemic thirst and its satiation are controlled by a hypothalamic circuit different from the one that controls osmotic thirst. When fluid volume drops, the kidneys send a hormone signal (angiotensin) that stimulates midline hypothalamic neurons. These neurons, in turn, stimulate drinking.
Controlling Sexual Behavior
Individuals must feed and drink continually to survive. This is the essence of regulatory behavior. But notwithstanding procreation, which is essential to the survival of the species, sexual behavior is nonregulatory: it is not essential for the individual organism’s survival. That fact does nothing to convince most of us that sex is unimportant.
In Sigmund Freud’s psychodynamic theory, sexual drives are central to human behavior. Sexual themes repeatedly appear in our art, literature, and films. They bombard us via advertising and other sales pitches. Such significance makes it all the more important to understand the control of human sexual behavior in terms of both gonadal hormones and brain circuits.
Effects of Sex Hormones on the Brain
During the fetal stage of prenatal development, a male’s Y chromosome controls the differentiation of embryonic gonad tissue into testes, which in turn secrete testosterone. This process is an organizing effect of gonadal hormones. Testosterone masculinizes both the sex organs and the brain during development. A major organizing effect of gonadal hormones on the brain is in the hypothalamus, especially the preoptic area of the medial hypothalamus. Organizing effects also operate in other nervous system regions, notably the amygdala, the prefrontal cortex, and the spinal cord.
The case study in Section 3-3 describes such epigentic inheritance.
Gonadal hormones produce enzymes necessary for epigenetic changes such as gene methylation. One action of steroid hormones is to methylate brain regions. For example, estrogen methylates the preoptic area of females, leading to the suppression of male characteristics. Growing evidence indicates that increased environmental levels of compounds, such as agricultural pesticides, can interfere with hormone activity, resulting in multigenerational epigenetic effects (e.g., McCarthy et al., 2009). These effects may be linked to anxiety levels and obesity and possibly to the organizational effects of gonadal hormones.
Sex-
Hormonal actions on the adult brain are referred to as activating effects, in contrast with developmental organizing effects. Next we consider both, separately.
ORGANIZING EFFECTS OF SEX HORMONES During fetal development, a male’s testes produce male hormones, the androgens. In the developing rat, androgens are produced during the last week of fetal development and the first week after birth. The androgens produced at this time greatly alter both neural structures and later behavior. For example, a male rat’s hypothalamus and prefrontal cortex differ structurally from those of both female rats and of males that were not exposed to androgens during development.
Section 8-4 explains organizing influences of gonadal hormones and critical periods in brain development.
Males with little exposure to the androgen testosterone during development behave like genetically female rats in adulthood. If given estrogen and progesterone, they become sexually receptive and display typical female behaviors when mounted by males. Male rats castrated in adulthood do not act in this way.
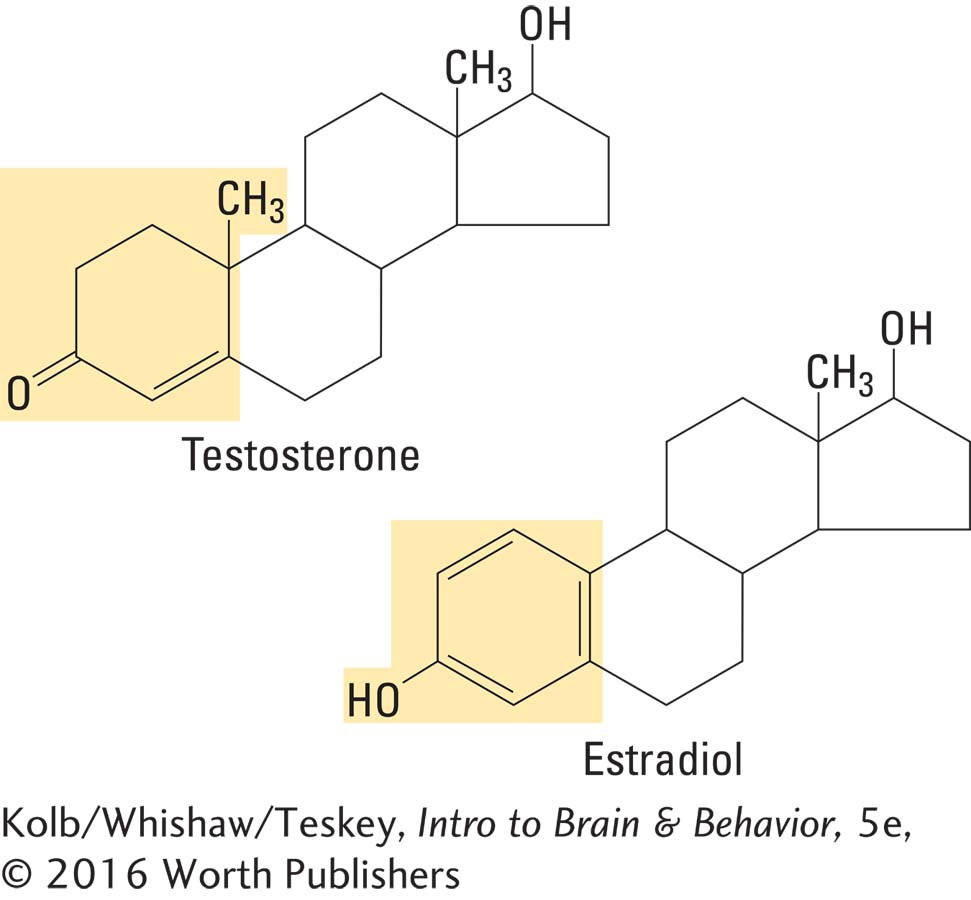
Sexual dimorphism, the differential development of brain areas in the two sexes, arises from a complex series of steps. Cells in the brain produce aromatase, an enzyme that converts testosterone into estradiol, one of the class of female sex hormones called estrogens. That is, when males produce testosterone, the brain converts it to an estrogen. Thus a female hormone, estradiol, actually masculinizes the male brain.
Females are not masculinized by the presence of estrogens because fetuses of both sexes produce a liver enzyme (alpha fetoprotein) that binds to estrogen, rendering it incapable of entering neurons. Testosterone is unaffected by alpha fetoprotein: it enters neurons and is converted into estradiol.
The organizing effects of testosterone are clearly illustrated in the preoptic area of the hypothalamus (see Figure 12-12A), which plays a critical role in male rats’ copulatory behavior. Comparing this area in males and females, Roger Gorski (1984) and his colleagues found a nucleus about five times as large in the males as in the females. Significantly, manipulating gonadal hormones during development can alter the sexual dimorphism of the preoptic area. Castrating male rats at birth leads to a smaller preoptic area; treating infant females with testosterone enlarges it.
The organizing effects of gonadal hormones are more difficult to study in humans. However, John Money and Anke Ehrhardt (1972) revealed an important role of these hormones in human development. Clinical Focus 12-5, Androgen Insensitivity Syndrome and Androgenital Syndrome, describes this role.
12-5
Androgen Insensitivity Syndrome and the Androgenital Syndrome
After the testes have formed in a male fetus, sexual development depends on the actions of testicular hormones. Studying people with androgen insensitivity syndrome makes this dependence crystal clear. In this syndrome, an XY (genetic male) fetus produces androgens, but the body cannot to respond to them.
Because androgen insensitivity syndrome does not affect estrogen receptors, these people are still responsive to estrogen produced by both the adrenal glands and the testes. As a result, they develop female secondary sexual characteristics during puberty, even without additional hormone treatment. A person with androgen insensitivity syndrome is therefore a genetic male who develops a female phenotype, that is, appears to be female, as shown in the photograph on the left.
If no Y chromosome is present to induce the growth of testes, an XX (genetic female) fetus develops ovaries and becomes a female. If the adrenal glands of either the mother or the infant produces an excessive amount of androgens, however, exposure of the female fetus to them produces the androgenital syndrome (congenital adrenal hyperplasia).
The effects vary, depending on when the androgens are produced and on the level of exposure. In extreme cases, the clitoris enlarges until it can be mistaken for a small penis, as shown in the photograph on the right.
In less severe cases, no gross change in genital structure develops, but there is a behavioral effect: these girls show a high degree of tomboyishness. In early childhood, they identify with boys and prefer boys’ clothes, toys, and games. One explanation for this behavioral effect is that the developing brain is masculinized, which changes later behavior.
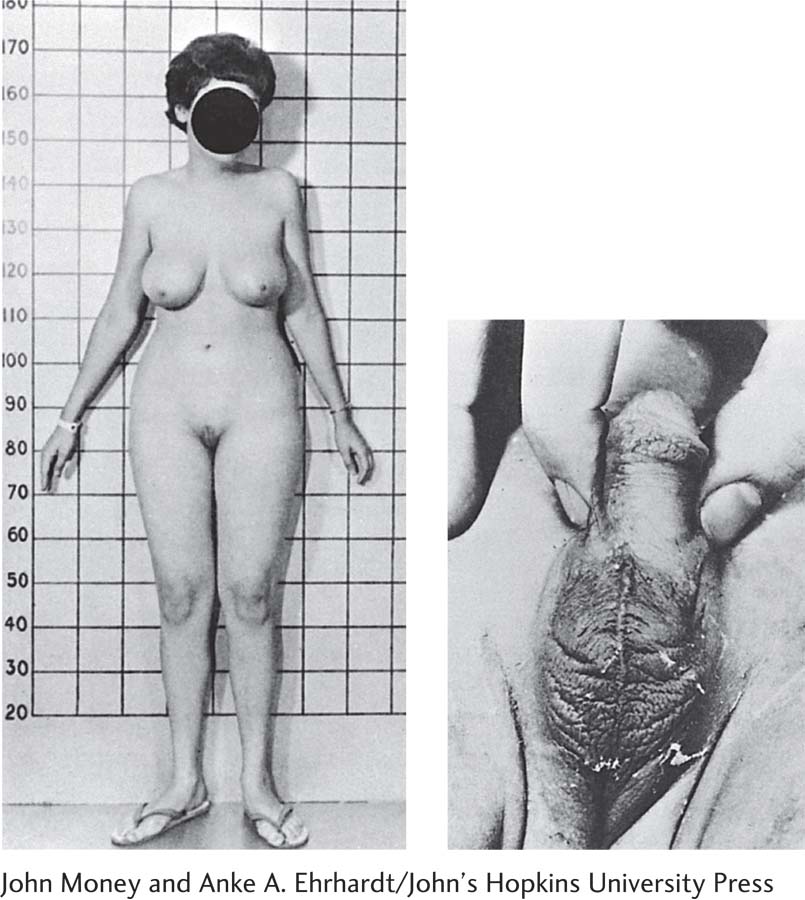
John Money and Anke A. Ehrhardt/John’s Hopkins University Press
Section 6-5 details broader activating effects of sex hormones on male and female behavior.
ACTIVATING EFFECTS OF SEX HORMONES The sexual behavior of both males and females also depends on the actions of gonadal hormones on the adult brain. In most vertebrate species, female sexual behavior varies in the course of an estrous cycle, during which the levels of ovarian hormones fluctuate. The rat’s estrous cycle is about 4 days long, with sexual receptivity occurring only in the few hours during which the production of the ovarian hormones estrogen and progesterone peaks. These ovarian hormones alter brain activity, which in turn alters behavior. In female rats, various chemicals released after mating inhibit further mating behavior.
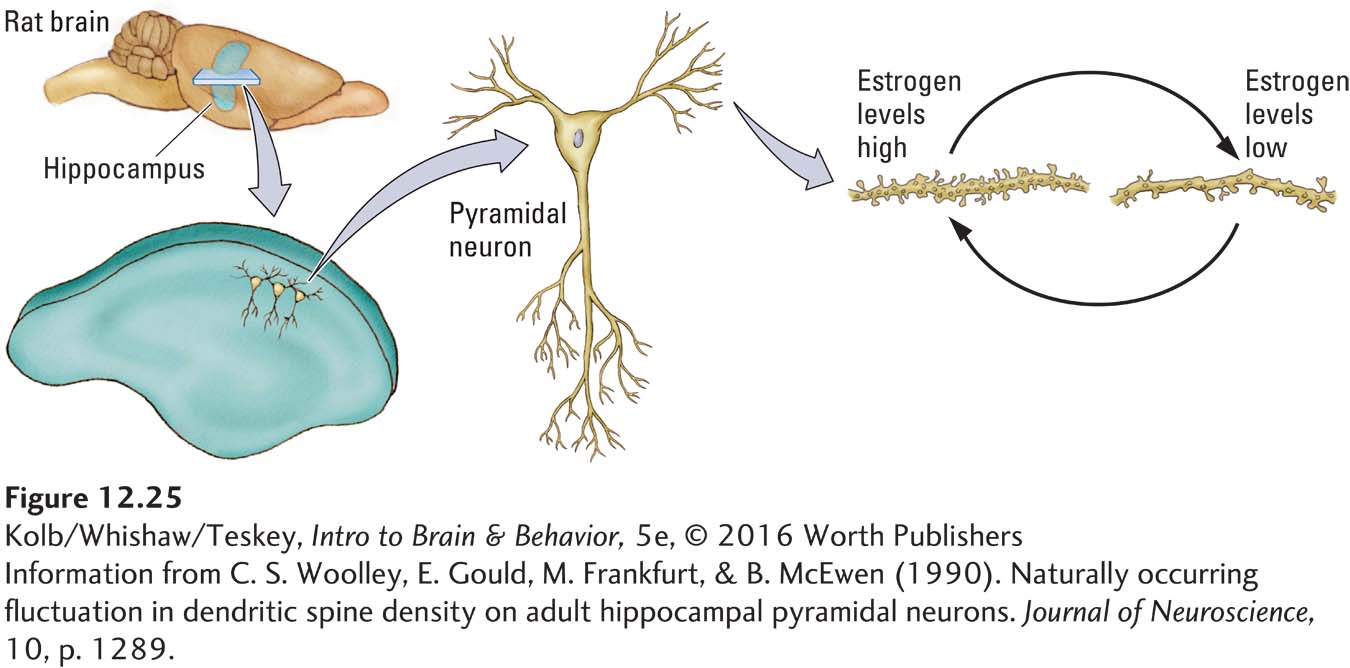
The activating effect of ovarian hormones can be seen clearly in hippocampal cells. Figure 12-25 compares hippocampal pyramidal neurons taken from female rats at two points in the estrous cycle: one when estrogen levels are high and the other when they are low. When estrogen levels are high, more dendritic spines and presumably more synapses emerge. These neural differences during the estrous cycle are all the more remarkable when we consider that cells in the female hippocampus are continually changing their connections to other cells every 4 days throughout the animal’s adulthood.
In males, testosterone activates sexual behavior in two distinct ways. First, testosterone’s actions on the amygdala are related to the motivation to seek sexual activity. Second, the actions of testosterone on the hypothalamus are needed to produce copulatory behavior. We look at both processes next.
Hypothalamus, Amygdala, and Sexual Behavior
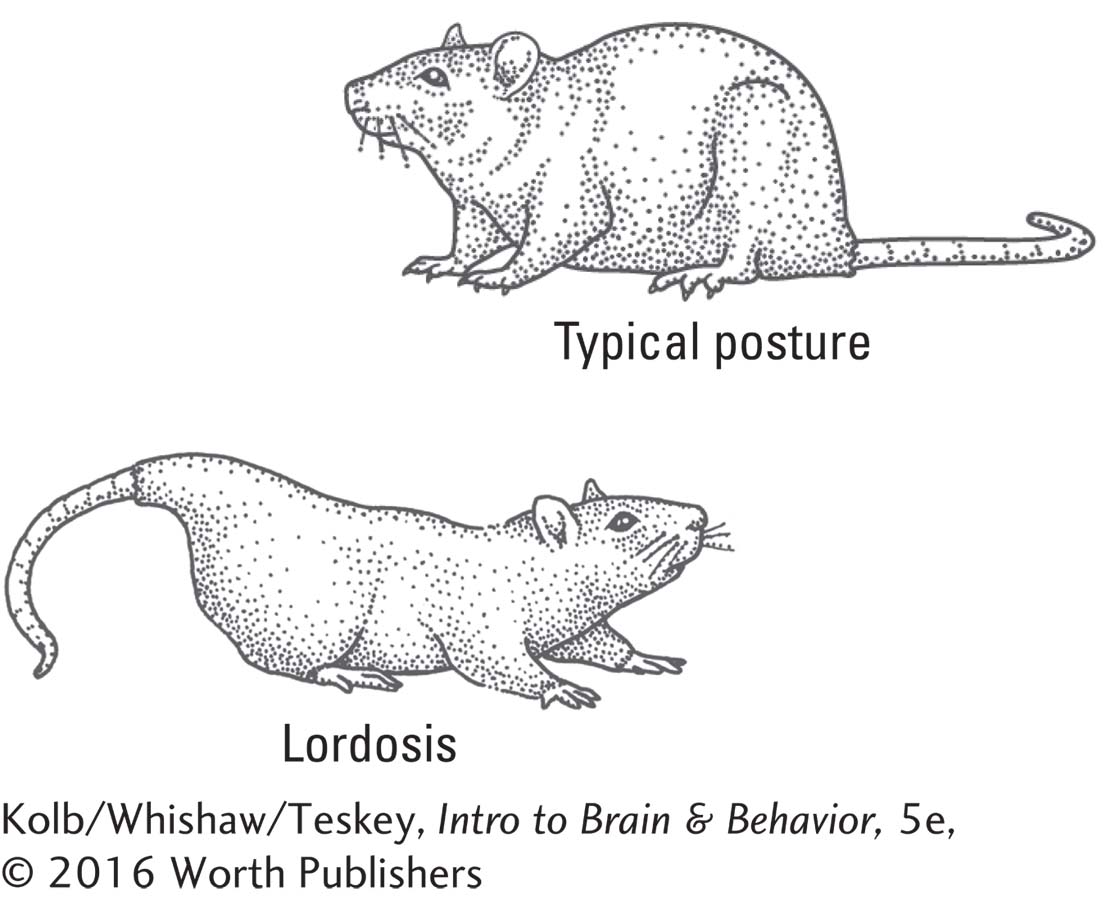
The hypothalamus is the critical structure controlling copulatory behaviors in both male and female mammals. The ventromedial hypothalamus controls the female mating posture, which in quadrupedal animals is called lordosis: arching the back and elevating the rump while the female otherwise remains quite still. Damage to the VMH abolishes lordosis. The role of the VMH is probably twofold: it controls the neural circuit that produces lordosis, and it influences hormonal changes in the female during coitus.
In males, neural control of sexual behavior is somewhat more complex. The medial preoptic area, which is larger in males than in females, controls copulation. Damage to this area greatly disrupts mating performance, whereas its electrical stimulation activates mating, provided that testosterone is circulating in the bloodstream. Curiously, although destruction of the medial preoptic area stops male mammals from mating, they continue to show interest in receptive females. For instance, monkeys with lesions in the medial preoptic area will not mate with receptive females, but they will masturbate while watching them from across the room.
Barry Everitt (1990) designed an ingenious apparatus that allows male rats to press a bar to deliver receptive females. After males were trained to use this apparatus, shown in Figure 12-26, lesions were made in their medial preoptic areas. Immediately, their sexual behavior changed. They would still press the bar to obtain access to females but would no longer mate with them.

Barry J. Everitt, Department of Experimental Psychology and the MRC-
Apparently, the medial preoptic area controls mating but not sexual motivation. The brain structure responsible for motivation appears to be the amygdala. When Everitt trained male rats in the apparatus and then lesioned their amygdala, they would no longer press the bar to gain access to receptive females, but they would mate with receptive females provided to them.
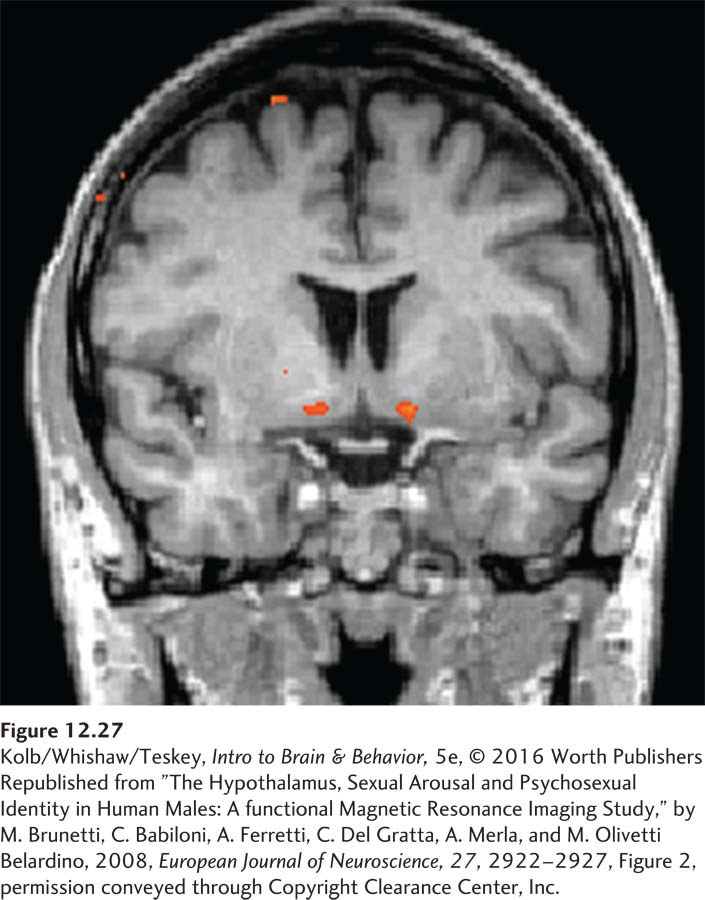
It is not practical to discriminate small hypothalamic nuclei in fMRI studies of humans. Studies have shown a bilateral increase in hypothalamic activity when men view erotic video clips but not when they view sports video clips (Figure 12-27). The degree of sexual arousal is related to the increase in hypothalamic activity (e.g., Brunetti et al., 2008).
In summary, the hypothalamus controls copulatory behavior in both male and female mammals. In males, the amygdala influences sexual motivation and probably plays a key role in female sexual motivation as well, especially among females of species, such as humans, whose sexual activity is not tied to fluctuations in ovarian hormones.
Sexual Orientation, Sexual Identity, and Brain Organization
Does sexual orientation—a person’s sexual attraction to the opposite sex or to the same sex or to both sexes—
Indeed, it appears virtually impossible to change a person’s sexual orientation. Lesbian couples commonly rear heterosexual children, and no evidence supports the notion that homosexuality is a lifestyle choice or that it is an effect of social learning (Bao & Swaab, 2011). The place to look for differences, therefore, is in the brain of people who identify themselves as heterosexual and homosexual.
Like rats, humans have sex-
But sex differences in the brain are not simply a matter of hormones. Epigenetics plays a role too, beginning early in development. In females, for example, one of the two X chromosomes is largely silenced, but not all its genes are silenced, which provides a basis for sex differences. Furthermore, emerging evidence suggests that sex differences in the hypothalamus result from differences in gene methylation (for a review, see McCarthy and coworkers, 2009).
Among homosexuals, variations in epigenetic effects could lead to differences in the architecture and function of the hypothalamus. Differences in the hypothalamus of heterosexual and homosexual men suggest that homosexual men form, in effect, a third sex because their hypothalamus differs from that of both females and heterosexual males.
Architectural and functional differences in the hypothalamus may form a basis for the spectrum of gender identity—a person’s degree of feeling male or female. People who view themselves as transgender, whose personal characteristics transcend traditional gender boundaries and corresponding sexual norms, believe they were born the wrong sex. Their desire to live as a member of the other sex can be so strong that they undergo sex change surgery.
More evidence favoring a neural basis for gender identity, including transgender identity, in Section 15-5.
Several biological factors appear to influence the likelihood of transgender identity—
In summary, differences in sexual orientation and gender identity appear to result from prenatal events that influence the organization and function of the brain, not from postnatal social or environmental experiences.

Cognitive Influences on Sexual Behavior
People think about sex, dream about sex, make plans about sex. These behaviors may include activity in the amygdala or the hypothalamus, but they must certainly also include the cortex. This is not to say that the cortex is essential for sexual motivation and copulation.
In studies of rats whose entire cortex has been removed, both males and females still engage in sexual activity, although the males are somewhat clumsy. Nevertheless, the cortex must play a role in certain aspects of sexual behavior. For instance, imagery about sexual activity must include activity in the cortical ventral visual pathway. And thinking about sexual activity and planning for it must require frontal lobe participation.
As you might expect, these aspects of sexual behavior are not easily studied in rats, and they remain uncharted waters in research on humans. However, changes in the sexual behavior of people with frontal lobe injury are well documented. And recall J. P.’s case, described in Clinical Focus 12-2. Although J. P. was uninhibited in his sexual behavior, frontal lobe damage is just as likely to produce a loss of libido (sexual interest). The wife of a man who 5 years earlier had a small tumor removed from the medial frontal region complained that she and her husband had since had no sexual contact whatever. He was simply not interested, even though they were both still in their twenties.
The husband said that he no longer had sexual fantasies or sexual dreams, and although he still loved his wife, he did not have any sexual urges toward her or anyone else. Such cases clearly indicate that the human cortex is important in controlling sexual behaviors. The exact nature of its role remains poorly understood.
12-5 REVIEW
Control of Regulatory and Nonregulatory Behavior
Before you continue, check your understanding.
Question 1
The three main hypothalamic regions that control feeding are _________________, _________________, and _______________.
Question 2
The key structures in the control of sexual behavior are the _______________ and the _______________.
Question 3
The two types of effects that hormones exert on the brain are __________________ and _________________.
Question 4
___________________ thirst results from an increase in the concentration of dissolved chemicals; ______________ thirst results from a decline in the total volume of body fluids.
Question 5
Describe why sex differences in the brain are not simply a matter of hormones.
Answers appear in the Self Test section of the book.