3-3 Genes, Cells, and Behavior
Your genotype (genetic makeup) influences your physical and behavioral traits, which combine to form your phenotype (individual characteristics). Genetic analysis conducted by the Human Genome Project has cataloged the human genome—
Figure 1-12 shows how a Neanderthal woman might have looked.
Researchers have succeeded in sequencing the long-
Studying how genes influence our traits is the objective of Mendelian genetics, named for Gregor Mendel, whose research led to the concept of the gene. Studying how the environment influences gene expression is the objective of epigenetics. In this section we describe how both factors influence our phenotypes.
Mendelian Genetics and the Genetic Code
The nucleus of each human somatic cell contains 23 pairs of chromosomes, or 46 in all. One member of each pair comes from the mother, and the other member comes from the father. The chromosome pairs are numbered from 1 to 23, roughly according to size, with chromosome 1 being the largest (Figure 3-20).
Chromosome pairs 1 through 22 are called autosomes, and they contain the genes that contribute most to our physical appearance and behavior. The twenty-
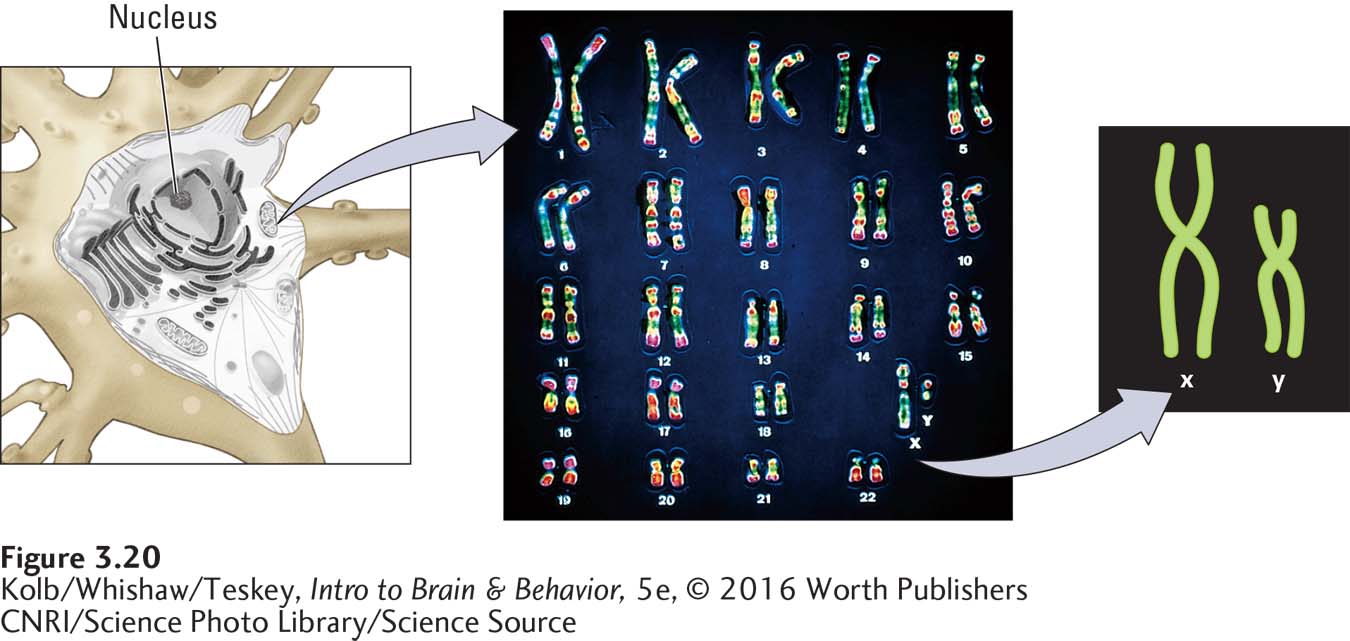
Because all but your sex chromosomes are matched pairs, each cell contains two copies of every gene, one inherited from your mother, the other from your father. These two copies of a gene are called alleles. The term matched here does not necessarily mean identical. The nucleotide sequences in a pair of alleles may be either identical or different. If they are identical, the two alleles are homozygous (homo-
The nucleotide sequence most common in a population is called the wild-
Dominant and Recessive Alleles
If both alleles in a gene pair are homozygous, the two encode the same protein, but if the two alleles in a pair are heterozygous, they encode somewhat different proteins. Three possible outcomes attend the heterozygous condition when these proteins express a physical or behavioral trait: (1) only the allele from the mother may be expressed, (2) only the allele from the father may be expressed, or (3) both alleles may be expressed simultaneously.
A member of a gene pair that is routinely expressed as a trait is called a dominant allele; a unexpressed allele is recessive. Alleles can vary considerably in their dominance. In complete dominance, only the allele’s own trait is expressed in the phenotype. In incomplete dominance, the allele’s own trait is expressed only partially. In codominance, both the allele’s own trait and that of the other allele in the gene pair are expressed completely.
Each gene makes an independent contribution to the offspring’s inheritance, even though the contribution may not always be visible in the offspring’s phenotype. When paired with a dominant allele, a recessive allele is often not expressed. Still, it can be passed on to future generations and influence their phenotypes when not masked by the influence of some dominant trait.
Genetic Mutations
The mechanism described in Section 3-2 for reproducing genes and passing them on to offspring is fallible. Errors can arise in the nucleotide sequence when reproductive cells make gene copies. The altered alleles are mutations.
A mutation may be as small as a change in a single nucleotide base, or single nucleotide polymorphism (SNP, pronounced snip). This one base change results in a change in a codon and a resulting change in one amino acid in a protein. A single amino acid change is a mutation and is often sufficient to alter the protein’s function.
Because the average gene has more than 1200 nucleotide bases, an enormous number of SNPs as well as more complex losses and changes in bases can occur on a single gene. For example, the BRCA1 (breast cancer) gene, found on chromosome 17, is a caretaker gene that contributes to preventing breast cancer and other cancers in both men and women. More than 1000 mutations of this gene have already been found. Thus, in principle, there are more than 1000 ways in which to inherit a predisposition to a cancer just from this gene.
A mutation in a nucleotide or the addition of a nucleotide to a gene sequence can be beneficial or disruptive or both. For example, a SNP in which a T base is substituted for an A base in the HBB (hemoglobin) gene on chromosome 11 causes sickle-
Each of us carries a surprisingly large number of genetic mutations. Because of cell division, different mutations may be localized in different parts of our body and brain and may contribute to individual variations in our organs, including the brain (Charney, 2012). While mutations may be beneficial or seemingly neutral to the functioning of the organism that carries them, most mutations have negative effects. If not lethal, they produce in their carrier debilitating physical and behavioral abnormalities.
Neuroscientists cannot yet explain human behavior in relation to genes and neurons, but we know the severe behavioral consequences of about 2000 genetic abnormalities that affect the nervous system. For example, an error in a gene could produce a protein that should be an ion channel but will not allow the appropriate substance to pass. It may produce a pump that will not pump or a protein that the cell’s transportation system refuses to transport.
Applying Mendel’s Principles
Experiment 1-1 describes one of Mendel’s experiments.
Gregor Mendel introduced the concept of dominant and recessive alleles in the nineteenth century, when he studied pea plants. Today, scientists study genetic variation to gain insight into how genes, neurons, and behaviors are linked. This knowledge is directed toward explaining healthy behavior and helping reduce the negative effects of genetic abnormalities, perhaps someday even eliminating them.
Allele Disorders That Affect the Brain
Scientists Warren Tay and Bernard Sachs first described the disorder.
Some disorders caused by mutant genes illustrate Mendel’s principles of dominant and recessive alleles. One is Tay-
Symptoms usually appear a few months after birth and rarely at later ages. The baby begins to have seizures, deteriorating eyesight, and degenerating motor and mental abilities. Inevitably, the child dies within a few years. Tay-
The dysfunctional Tay-
Because both parents have survived to adulthood, both must also possess a corresponding dominant wild-
In any child born of two Tay-
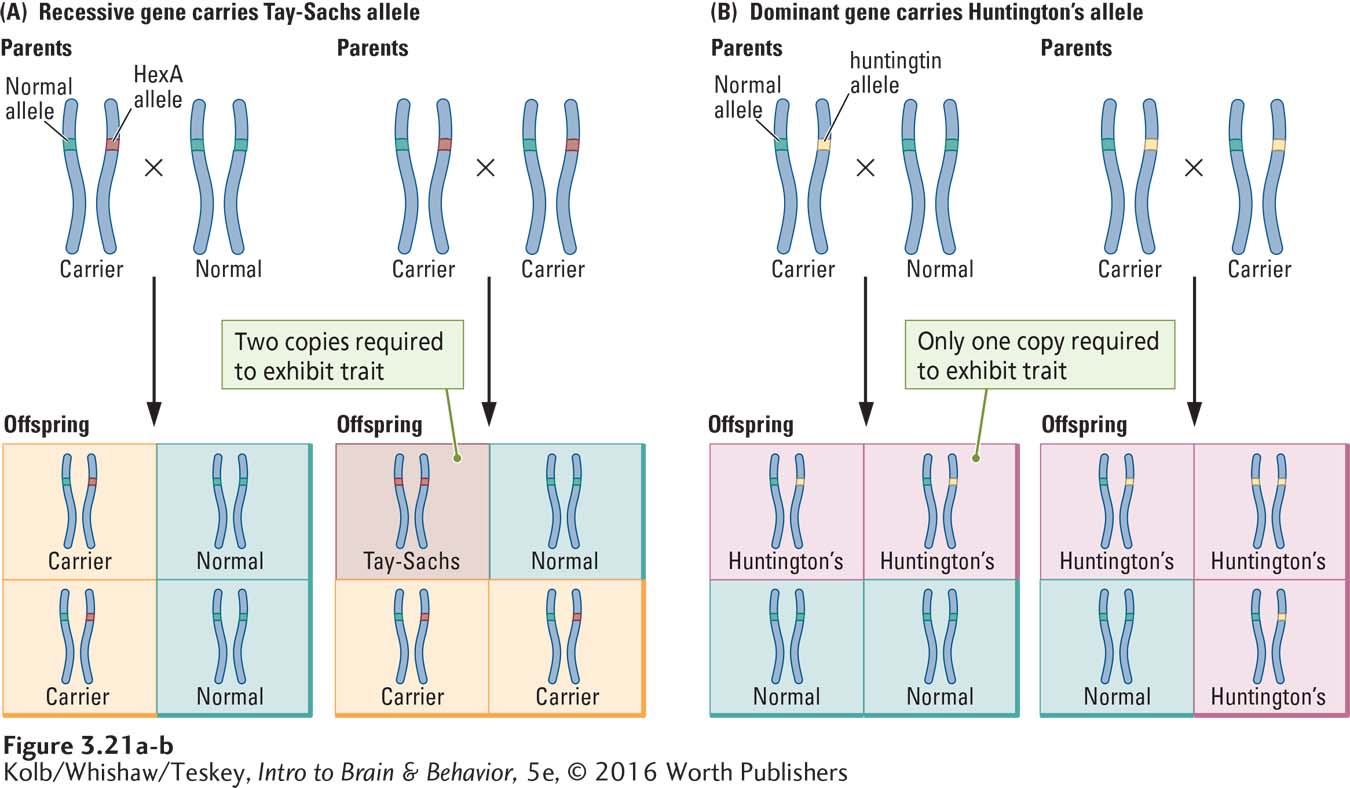
The chance of a child of two carriers being normal is 25 percent, the chance of being a carrier is 50 percent, and the chance of having Tay-
The Tay-
Fortunately, a blood test can detect whether a person carries the Tay-
The normal dominant allele that a carrier of Tay-
Symptoms can begin at any time from infancy to old age, but they most often start in midlife and include abnormal involuntary movements, which is why the disorder was once called a chorea (from the Greek meaning dance). Other symptoms are memory loss and eventually a complete deterioration of behavior, followed by death. The abnormal HTT (huntingtin) allele is dominant, the recessive allele normal, so only one defective allele is needed to cause the disease.
Figure 3-21B illustrates the inheritance patterns associated with a dominant allele on chromosome 4 that produces Huntington disease. If one parent carries the defective allele, offspring have a 50 percent chance of inheriting the disorder. If both parents have the defective allele, the chance of inheritance increases to 75 percent. As discussed further in Clinical Focus 3-4, Huntington Disease, on page 100, because the abnormal huntingtin allele usually is not expressed until midlife, after the people who possess it have had children, it is passed down even though it is lethal.
As with the allele causing Tay-
3-4
Huntington Disease
Woody Guthrie, whose protest songs made him a spokesman for farm workers during the Great Depression of the 1930s, is revered among the founders of American folk music. His best-
Guthrie died in 1967 after struggling with what was eventually diagnosed as Huntington disease. His mother had died of a similar condition, although her illness was never diagnosed. Two of Guthrie’s five children from two marriages developed the disease, and his second wife, Marjorie, became active in promoting its study.
Huntington disease is devastating, characterized by memory impairment; choreas (abnormal, uncontrollable movements); and marked changes in personality, eventually leading to nearly total loss of healthy behavioral, emotional, and intellectual functioning. Even before the onset of motor symptoms, Huntington disease impairs theory of mind, a person’s ability to assess the behavior of others (Eddy and Rickards, 2015).
The symptoms of Huntington disease result from neuronal degeneration in the basal ganglia and cortex. Symptoms can appear at any age but typically start in midlife. In 1983, the HTT (huntingtin) gene responsible for forming the abnormal huntingtin protein was found on chromosome 4.
The HTT gene has been a source of insights into the transmission of genetic disorders. Part of the gene contains repeats of the base sequence CAG. The CAG codon encodes the amino acid glutamine. If the number of CAG repeats exceeds about 40, then the carrier, with 40 or more glutamine amino acids in the huntingtin protein, has an increased likelihood of Huntington symptoms.
As the number of CAG repeats increases, the onset of symptoms occurs earlier in life, and the disease progresses more rapidly. Typically, non-
Investigations into why brain cells change in Huntington disease and into potential treatments use transgenic animal models. Mice, rats, and monkeys that have received the HTT gene feature the abnormal huntingtin protein and display symptoms of Huntington disease (Gu et al., 2015).

Chromosome Abnormalities
Genetic disorders are not caused solely by single defective alleles. Some nervous system disorders are caused by copy number variations, that is, aberrations in a part of a chromosome or even an entire chromosome. Copy number variations are related to a variety of disorders, including autism, schizophrenia, and learning disabilities. Often though, copy number variation has little obvious consequence or is beneficial. For example, humans average about 6 copies of the AMY1 (amylase) gene but may have as many as 15 copies. The gene is an adaptation that improves the ability to digest starchy foods (Mimori et al., 2015).
One condition due to a change in chromosome number in humans is Down syndrome, which affects approximately 1 in 700 children. Down syndrome is usually the result of an extra copy of chromosome 21. One parent (usually the mother) passes on two copies of chromosome 21 to the child rather than the normal single chromosome. Combining these two with one chromosome from the other parent yields three chromosomes 21, an abnormal number called a trisomy (Figure 3-22).

Although chromosome 21 is the smallest human chromosome, its trisomy can dramatically alter a person’s phenotype. People with Down syndrome have characteristic facial features and short stature. They are susceptible to heart defects, respiratory infections, and intellectual impairment. They are prone to developing leukemia and Alzheimer disease. Although people with Down syndrome usually have a much shorter than normal life span, some live to middle age or beyond. Improved educational opportunities for children with Down syndrome shows that they can learn to compensate greatly for their mental disabilities.
Genetic Engineering
Despite advances in understanding gene structure and function, the gap in understanding how genes produce behavior remains wide. To investigate gene structure and behavior relations, geneticists have invented methods to influence the traits genes express. This approach collectively defines the science of genetic engineering. In its simplest forms, genetic engineering entails manipulating a genome, removing a gene from a genome, or modifying or adding a gene to the genome. Its techniques include selective breeding, cloning, and transgenics.
Selective Breeding
The oldest means of influencing genetic traits is the selective breeding of animals and plants. Beginning with the domestication of wolves into dogs more than 30,000 years ago, humans have domesticated many animal species by selectively breeding males and females that display particular traits. The selective breeding of dogs, for example, has produced the species with the most diverse traits of all animal species: breeds that can run fast, haul heavy loads, retrieve prey, dig for burrowing animals, climb rocky cliffs in search of sea birds, herd sheep and cattle, or sit on an owner’s lap and cuddle. Selective breeding especially influences dogs’ sociability with humans (Persson et al., 2015).
Maintaining spontaneous mutations is one objective of selective breeding. Using this method, researchers produce whole populations of animals possessing some unusual trait that originally arose as an unexpected mutation in only one individual or in a few animals. In laboratory colonies of mice, for example, multiple spontaneous mutations have been discovered and maintained to produce over 450 different mouse strains.
Unlike other animals, humans can consent to experimental procedures. Section 7-7 frames debates on the benefits and ethics of conducting research using nonhuman animals.
Some strains of mice make abnormal movements, such as reeling, staggering, and jumping. Other strains have diseases of the immune system; others are blind or cannot hear. Some mice are smart; some mice are not; some have big brains;, some, small; and many display distinctive behavioral traits. Many such genetic variations can also be found in humans. As a result, the neural and genetic bases of the altered behavior in the mice can be studied systematically to understand and treat human disorders.
Cloning
Sections 7-1 and 7-5 review genetic methods used in neuroscience research.
More direct approaches to manipulating the expression of genetic traits include altering early embryonic development. One such method is cloning—producing an offspring that is genetically identical to another animal.
A team of researchers in Scotland cloned Dolly in 1996. As an adult, she mated and bore a lamb.
To clone an animal, scientists begin with a cell nucleus containing DNA, usually from a living animal donor, place it in an egg cell from which the nucleus has been removed, and after stimulating the egg to start dividing, implant the new embryo in the uterus of a female. Because each individual animal that develops from these cells is genetically identical to the donor, clones can be used to preserve valuable traits, to study the relative influences of heredity and environment, or to produce new tissue or organs for transplant to the donor. Dolly, a female sheep, was the first cloned mammal.
Cloning has matured from an experimental manipulation to a commercial enterprise. The first horse to be cloned was Charmayne James’s horse Scamper, the mount she rode to 11 world championships in barrel racing. The first cat to be cloned, shown in Figure 3-23, was called Copycat. The first rare species cloned was an Asian gaur, an animal related to the cow. Investigators anticipate that cloning will be used to reanimate extinct animals, a process called de-

Transgenic Techniques
Transgenic technology enables scientists to introduce genes into an embryo or remove genes from it. For example, introducing a new gene can enable cows or goats to produce medicines in their milk. The medicines can be extracted from the milk to treat human disease. Transgenic techniques used to take a mouse gene that affords resistance to tuberculosis and insert it into cows has increased their resistance to TB (Wu et al., 2015).
Chimeric animals are composites formed when an embryo of one species receives cells from a different species. The resulting animal has cells with genes from both parent species and behaviors that are a product of those gene combinations. The chimeric animal may display an interesting mix of the parent species’ behaviors. For example, chickens that received Japanese quail cells in early embryogenesis display some aspects of quail crowing behavior rather than chicken crowing behavior—
Chimerism is common in humans (Giorgi, 2015). Twin zygotes (fertilized eggs) may fuse into a single individual, twins may exchange cells through placental circulation, and the fetus and the mother may exchange cells with one another. Even organ transplant or stem cell recipients may incorporate transplanted cells into other organs.
In knock-
The neural basis of memory is the topic of Section 14-3.
Knockout technology is used to inactivate a gene, for example so that a line of mice fails to express it. The mouse line can then be examined to determine whether the targeted gene is responsible for a specific function or a human disorder and to examine possible therapies. It may be possible to knock out genes related to certain kinds of memory, such as emotional memory, social memory, or spatial memory. Knockout technology is a useful way of investigating the neural basis of memory as well as clinical conditions associated with learning impairments (Kusakari et al., 2015).
Phenotypic Plasticity and the Epigenetic Code
Our genotype is not sufficient to explain our phenotype. We all know that if we expose ourselves to the sun, our skin darkens; if we exercise, our muscles enlarge; if we study, we learn. Our phenotype also changes with our diet and as we age. In short, the extent of phenotypic variation, given the same genotype, is remarkable.
Every individual has a capacity to develop into more than one phenotype. This phenotypic plasticity is due in part to the genome’s capacity to express a large number of phenotypes and in part to epigenetics, the influence of environment and experience in phenotypic expression.
Seemingly puzzling features in the expression of genomes in relation to phenotypes are illustrated in strains of genetically identical mice, some of which develop a brain with no corpus callosum (Figure 3-24). The absence of this hemispheric connector results from an epigenetic influence on whether the trait is expressed in a particular mouse. It occurs in the embryo at about the time the corpus callosum should form. This lack of concordance (incidence of similar behavioral traits) is also observed in patterns of disease incidence in human identical twins, who share the same genome.
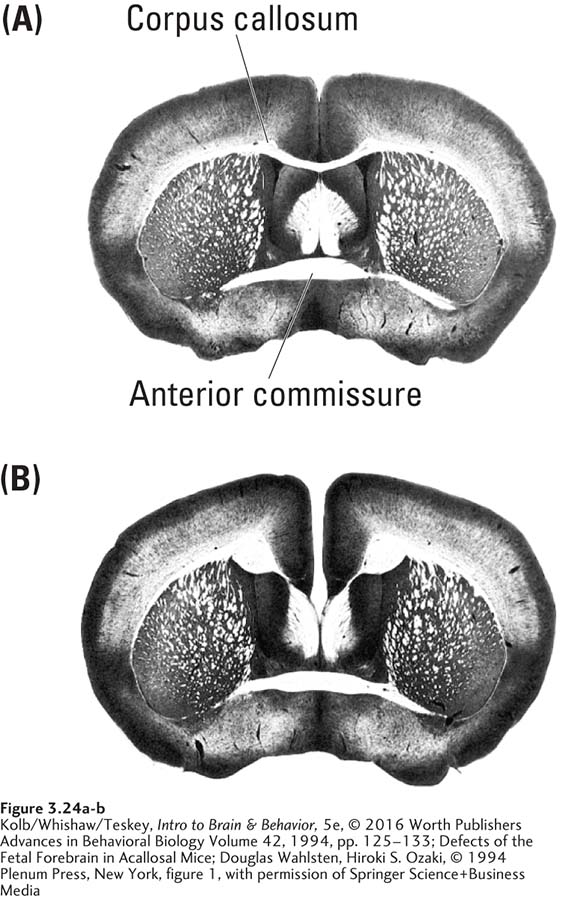
The concordance rate between identical twins for a vast array of diseases—
The cloned mice shown in Figure 2-1 exemplify phenotypic plasticity.
Phenotypic plasticity is in evidence not only in adult organisms but also in cells. In Section 3-1, we described the variety of neurons and glia found in the nervous system. Each of these cells usually has the same genotype. So also do the 248 other cell types of our body. How then do they become so different?
Applying the Epigenetic Code
The International Human Epigenome Consortium (IHEC) mandate is to describe the epigenetic code, as the Human Genome Project has described the genetic code.
The genes expressed in a cell are influenced by factors within the cell and in the cell’s environment. Once a fertilized egg begins to divide, each new cell finds itself in a somewhat different environment from that of its parent cell. The cell’s environment will determine which genes are expressed and so what kind of tissue it becomes, including what kind of nervous system cell it becomes. Environmental influences do not end at birth, of course. Our environment changes daily throughout our lives, as does its influence on our genes.
Epigenetic mechanisms create phenotypic variation without altering the base pair nucleotide sequence of the genes. Through these mechanisms, experience and the environment can allow a gene to be expressed or prevent its expression. Epigenetics is viewed as a second code; the first code is the genome. Epigenetics describes how a single genetic code produces each somatic cell type, explains how a single genome can code for many phenotypes, and describes how cell functions go astray to produce diseases ranging from cancer to brain dysfunction.
Epigenetic mechanisms can influence protein production either by blocking a gene to prevent transcription or by unlocking a gene so that it can be transcribed. This is where experiential and environmental influences come into play. To review, each of your chromosomes consists of a long, double-
Chromosomes are wrapped around supporting molecules of a protein called histone. Histone wrapping allows the many yards of a chromosome to be packaged in a small space, as yards of thread are wrapped around a spool. For any gene to be transcribed into messenger RNA, its DNA must be unspooled from the histones. Once unspooled, each gene must be instructed to transcribe mRNA. Then the mRNA must be translated into an amino acid chain that forms the protein. Figure 3-25 illustrates some ways that each step can be either enabled or blocked:
Methylation dramatically alters gene expression during brain development (see Sections 8-2 and 12-5) and can affect memory and brain plasticity (see Section 14-4).
Histone modification. DNA may unwrap or be stopped from unwrapping from the histone. At the top of Figure 3-25, a methyl group (CH3) or other molecule binds to the tails of histones to block DNA from unspooling. Its genes cannot be exposed for transcription with the block in place (left), but it can be opened for transcription (right) if the block is absent or removed.
Gene (DNA) methylation. Transcription of DNA into mRNA may be enabled or blocked. In Figure 3-25 at center, one or more methyl groups bind to CG base pairs to block transcription.
mRNA modification. mRNA translation may be enabled or blocked. In Figure 3-25, bottom, noncoding RNA (ncRNA) binds to mRNA, blocking translation.
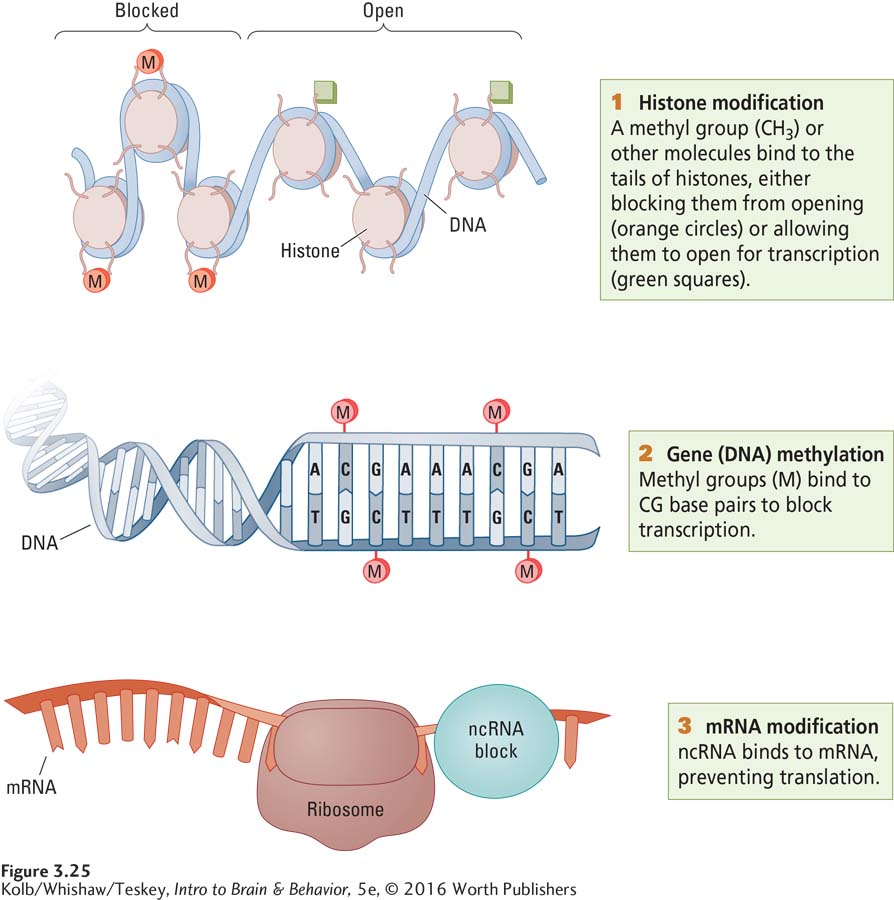
An environmental influence can either induce or remove one or more blocks, thus allowing the environment to regulate gene expression (Charney, 2012). It is through these epigenetic mechanisms that cells are instructed to differentiate into various body tissues and that our unique environment and experience induce changes in our brain that make us a unique individual. Some experientially induced events can also be passed from one generation to the next, as the following case study illustrates.
A Case of Inheriting Experience
The idea that traits are passed from parent to child through genes is a cornerstone of Mendelian genetics. Mendel’s theory also predicts that individual life experience cannot be inherited. Lars Olov Bygren and colleagues (Kaati et al., 2007) found, however, that individuals’ nutritional experiences can affect their offspring’s health.
The investigators focused on Norrbotten, a sparsely populated northern Swedish region. In the nineteenth century, Norrbotten was virtually isolated from the outside world. If the harvest there was bad, people starved. According to historical records, the years 1800, 1812, 1821, 1836, and 1856 saw total crop failure. The years 1801, 1822, 1828, 1844, and 1863 brought good harvests and abundance.
Bygren and colleagues identified at random individuals who had been subjected to famine or to plenty in the years just before they entered puberty. Then the researchers examined the health records and longevity of these people’s children and grandchildren.
The findings seem to defy logic. The descendants of the plenty group had higher rates of cardiovascular disease and diabetes and had a life expectancy more than seven years shorter than that of the famine group! Notably, these effects were found only in male offspring of males and female offspring of females.
Section 8-4 examines critical periods, limited time spans during which events have long-
Bygren and colleagues propose that diet during a critical period can modify the genetic expression of sex chromosomes—
Many other studies support Bygren and coworkers’ seminal findings. Together, this body of research makes a strong argument for epigenetics and for the idea that some epigenetic influences can be passed on for at least a few generations. Evidence that epigenetic influences play a demonstrable role in determining gene expression is disclosing how our experiences shape our brains to influence whom we become and how our current environment might influence our descendants’ epigenetic inheritance (Guerrero-
3-3 REVIEW
Genes, Cells, and Behavior
Before you continue, check your understanding.
Question 1
Each of our __________ chromosome pairs contains thousands of genes, and each gene contains the code for one __________.
Question 2
The genes we receive from our parents may include slightly different __________ of particular genes, which will be expressed in slightly different __________.
Question 3
Abnormalities in a gene, caused by a(n) __________, can result in an abnormally formed protein, hence in abnormal cell function. Chromosome abnormality can result in abnormal functioning of many genes. __________, for example, is caused by an extra copy of chromosome 21, a __________.
Question 4
Tay-
Question 5
__________ is the oldest form of genetic manipulation. Genetic engineering manipulates or alters the genome of an animal. __________ produces an animal that is genetically identical to a parent or sibling; __________ animals contain new, altered, or inactivated genes.
Question 6
__________ is an epigenetic mechanism that either enables or blocks transcription.
Question 7
What distinguishes Mendelian genetics from epigenetics?
Answers appear in the Self Test section of the book.