7-1 Measuring and Manipulating Brain and Behavior
During a lecture at a meeting of the Anthropological Society of Paris in 1861, Ernest Auburtin, a French physician, argued that language functions are located in the brain’s frontal lobes. Five days later a fellow French physician, Paul Broca, observed a brain-
Section 10-4 explores the anatomy of language and music and describes Broca’s contributions.
By 1863 Broca had collected eight similar cases and concluded that speech is located in the third frontal convolution of the left frontal lobe—
Linking Neuroanatomy and Behavior
At the beginning of the twentieth century, neuroanatomy’s primary tools were histological: brains were sectioned postmortem and the tissue (histo- in Greek) stained with various dyes. As shown in Figure 7-1, staining sections of brain tissue can identify cell bodies in the brain viewed with a light microscope (A), and selectively staining individual neurons reveals their complete structure (B). An electron microscope (C) makes it possible to view synapses in detail.
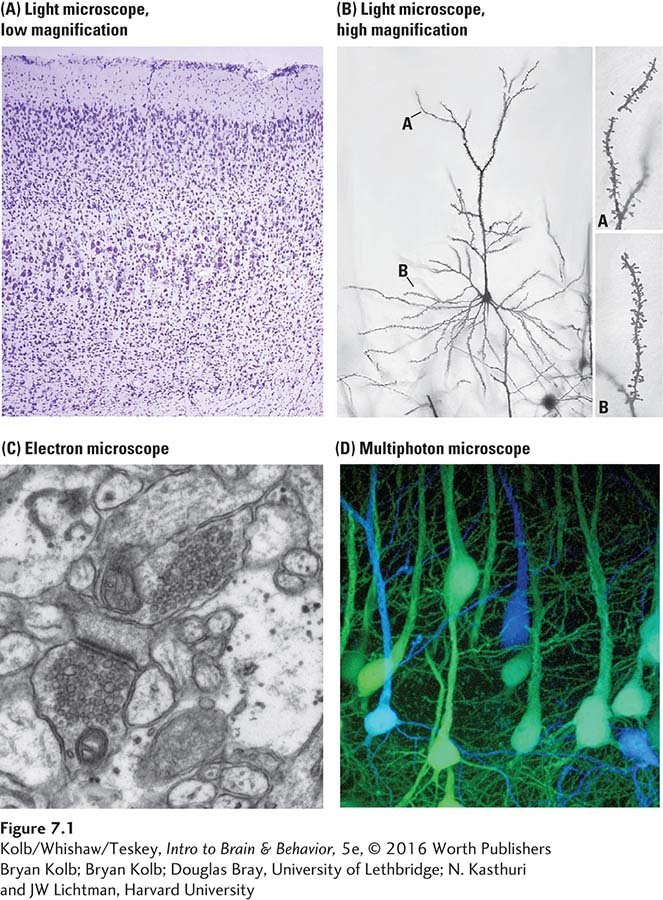
Bryan Kolb
Douglas Bray, University of Lethbridge
N. Kasthuri and JW Lichtman, Harvard University
Compare Brodmann’s map of the cortex, based on staining, shown in Figure 2-23.
By 1905, light microscopic techniques allowed researchers such as Korbinian Brodmann to divide the cerebral cortex into many distinct zones based on the characteristics of neurons in those zones. Investigators presumed that cortical zones had specific functions. By the dawn of the twenty-
Connections between anatomy and behavior can be seen in studies of animals trained on various types of learning tasks, such as spatial mazes. Such learning can be correlated with a variety of neuronanatomical changes, such as modifications in the synaptic organization of cells in specific cortical regions—
Corticosterone, a steroid hormone secreted in times of stress, is important in protein and carbohydrate metabolism (see Section 6-5).
Although changes in synaptic organization or cell survival have not yet been proved to be the basis of the new learning, experimental evidence reveals that preventing the growth of new hippocampal neurons leads to memory deficits. To test the idea that hippocampal neurons contribute to memory formation, researchers tested healthy rats and ADX rats—
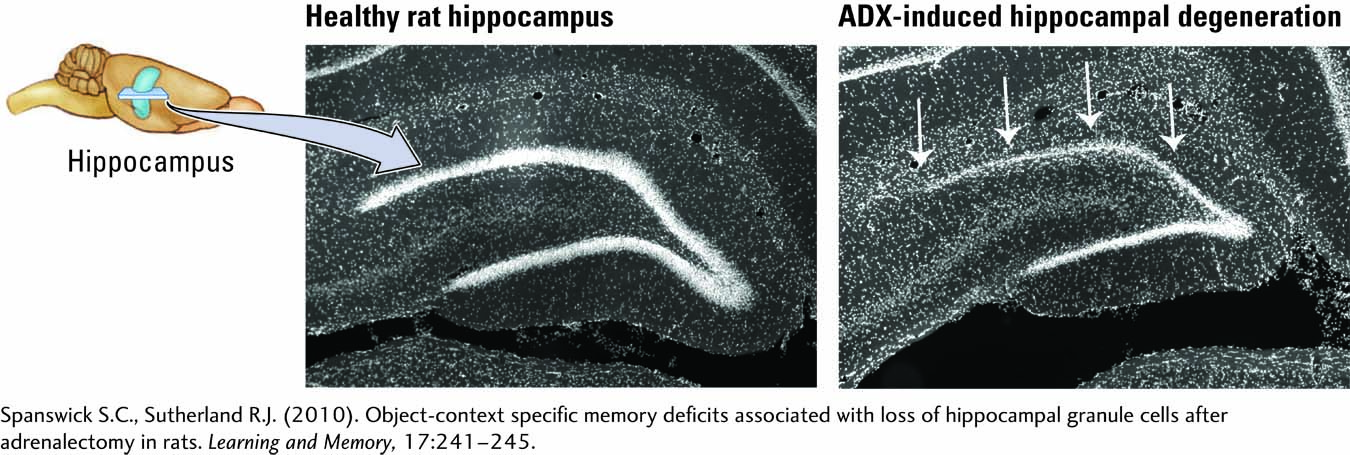
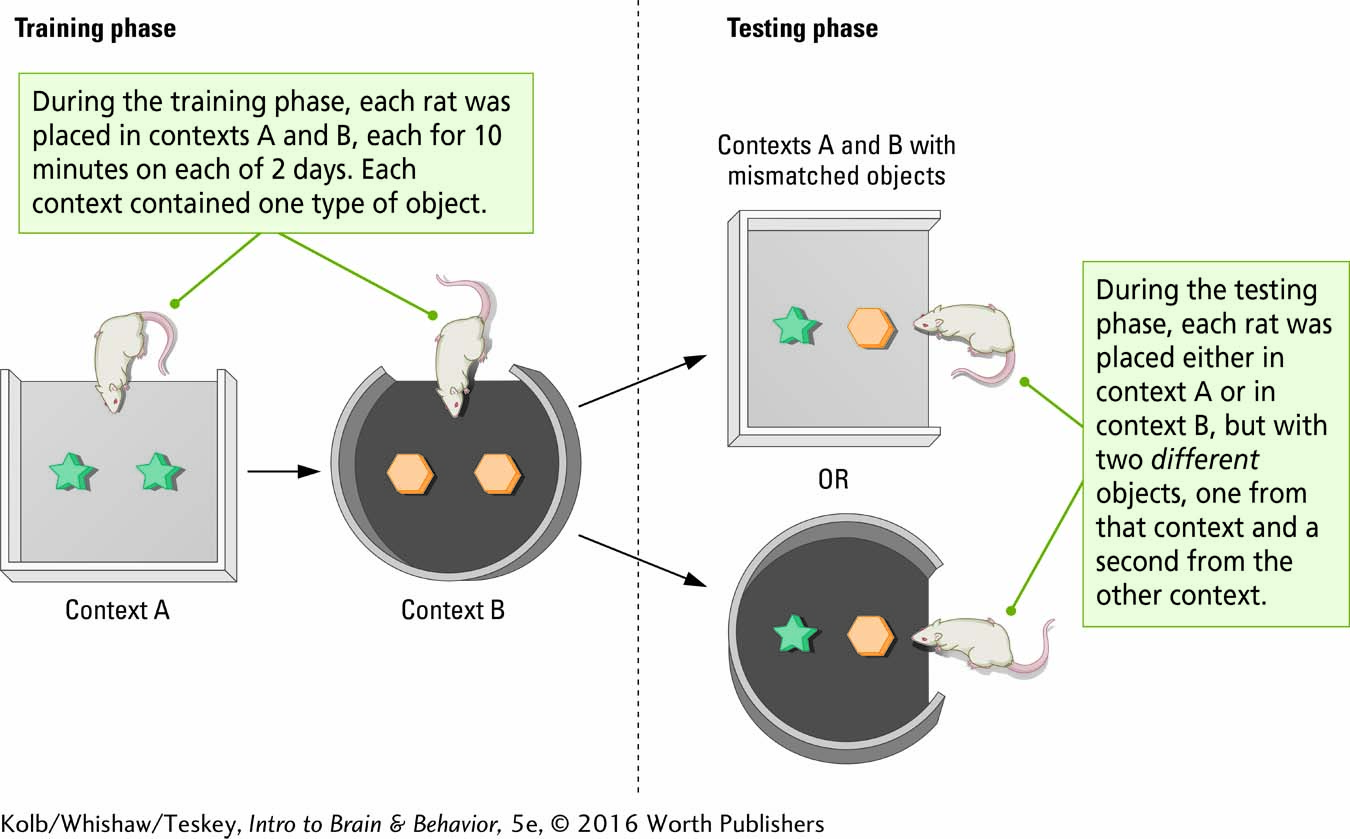
Procedure 1 in Experiment 7-1 contrasts the appearance of a healthy rat hippocampus (left) and neuronal degeneration in an ADX rat after surgery (right). The behavior of healthy and ADX rats was studied in the object–
As noted in the Results section of the experiment, when healthy rats encounter objects in the correct context, they spend little time investigating because the objects are familiar. If, however, they encounter an object in the wrong context, they are curious and spend about three-
EXPERIMENT
Question: Do hippocampal neurons contribute to memory formation?
Procedure 1
Rat hippocampus before (left) and after (right) surgical removal of the adrenal glands. Arrows point to neuronal degeneration resulting from a lack of corticosterone.
Procedure 2
The behavior of healthy and ADX rats was studied in an object–
Results
Healthy rats investigate the mismatch object more than the object that is in context, but the ADX rats performed at chance. The rats in another ADX group were given treatments known to increase neuron generation in the hippocampus (enriched housing and exercise in running wheels). The rats with hippocampal regeneration were not impaired at the mismatch task.
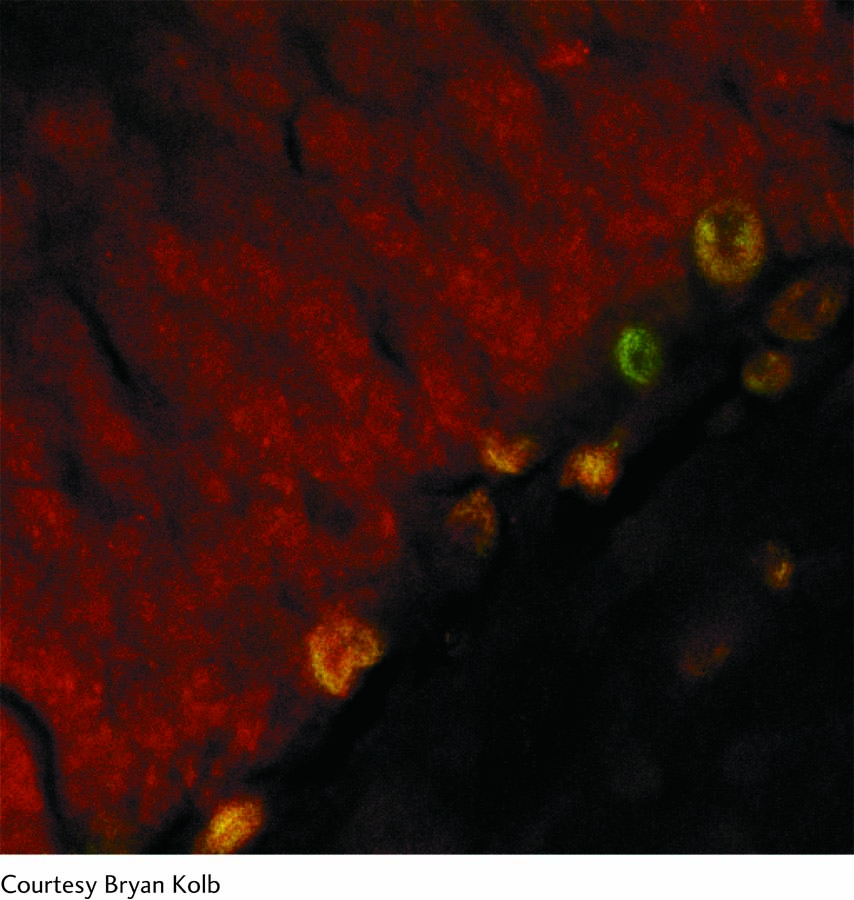
The confocal photo at right shows a rat hippocampus. A specific stain was used to identify new neurons, which appear yellow.
Conclusion: Hippocampal neurons are necessary for the contextual learning.
Information from S. Spanswick & R. J. Sutherland (2010). Object/context-
Another group of ADX rats given treatment known to increase neuron generation in the hippocampus—
Methods of Behavioral Neuroscience
The brain’s ultimate function is to produce behavior (movement). It follows that brain dysfunction should alter behavior in some way. The study of brain–
A major challenge to behavioral neuroscientists is developing methods for studying both typical and atypical behavior. Measuring behavior in humans and laboratory animals differs in large part because humans speak: investigators can ask them about their symptoms. It is also possible to use both paper-
Measuring behavior in laboratory animals is more complex. Researchers must learn to speak “ratese” with rat subjects or “monkeyese” with monkeys. In short, researchers must develop ways to enable the animals to reveal their symptoms. The development of the fields of animal learning and ethology, the objective study of animal behavior, especially under natural conditions, provided the basis for modern behavioral neuroscience (see Whishaw & Kolb, 2005). To illustrate the logic of behavioral neuroscience, we describe some measurement tools used with humans and some used with rats.
Neuropsychological Testing of Humans
The brain has exquisite control of an amazing array of functions ranging from movement control and sensory perception to memory, emotion, and language. As a consequence, any analysis of behavior must be tailored to the particular function(s) under investigation. Consider the analysis of memory.
Sections 14-1 through 14-3 analyze the varied categories of memory.
People with damage to the temporal lobes often complain of memory disturbance. But memory is not a single function; rather, multiple as well as independent memory systems exist. We have memory for events, colors, names, places, and motor skills, among other categories, and each must be measured separately. It would be rare indeed for someone to be impaired in all forms of memory. Neuropsychological tests of three distinct forms of memory are illustrated in Figure 7-2.
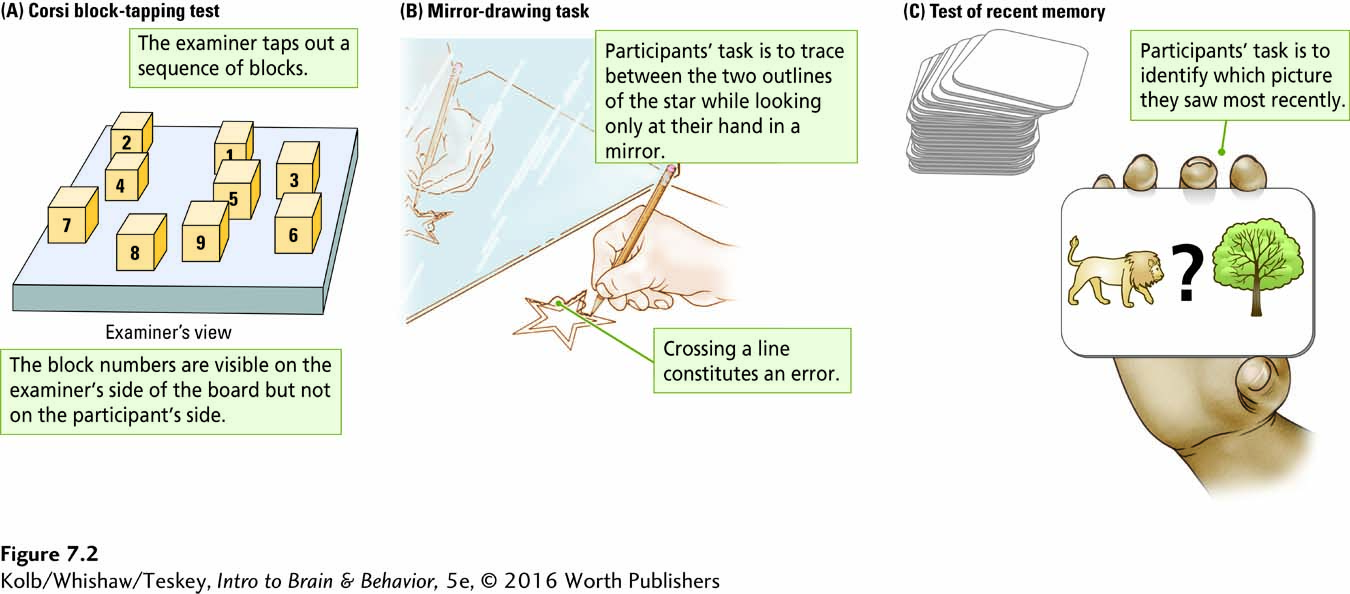
The Corsi block-
The Corsi test provides a measure of short-
Span + 1 identifies a different form of memory from block span. Different types of neurological dysfunction interfere differentially with tasks that superficially appear quite similar. Block span measures the short-
In this book, we refer to healthy human volunteers in research studies as participants and to people or animals that have a brain or behavioral impairment as patients or subjects.
The mirror-
In the recency memory task (Figure 7-2C), participants are shown a long series of cards, each bearing two stimulus items that are words or pictures. On some trials a question mark appears between the items. The subjects’ task is to indicate whether they have seen the items before and if so, which item they saw most recently. People may be able to recall that they have seen items before but be unable to recall which was most recent. Conversely, they may not be able to identify the items as being familiar, but when forced to choose the most recent one, they can identify it correctly.
Focus 15-4 compares effects of injuries to particular brain regions on performance of particular neuropsychological tasks.
The latter, counterintuitive result reflects the need for behavioral researchers to develop ingenious ways of identifying memory abilities. It is not enough simply to ask people to recall information verbally, although this too measures a form of memory.
Behavioral Analysis of Rats
Over the past century, psychologists interested in the neural basis of memory have devised a vast array of mazes and other tests and tasks to investigate different forms of memory in laboratory animals. Figure 7-3 illustrates three tests based on a task devised by Richard Morris in 1980. Researchers place rats in a large swimming pool where an escape platform lies just below the water surface, invisible to the rats.
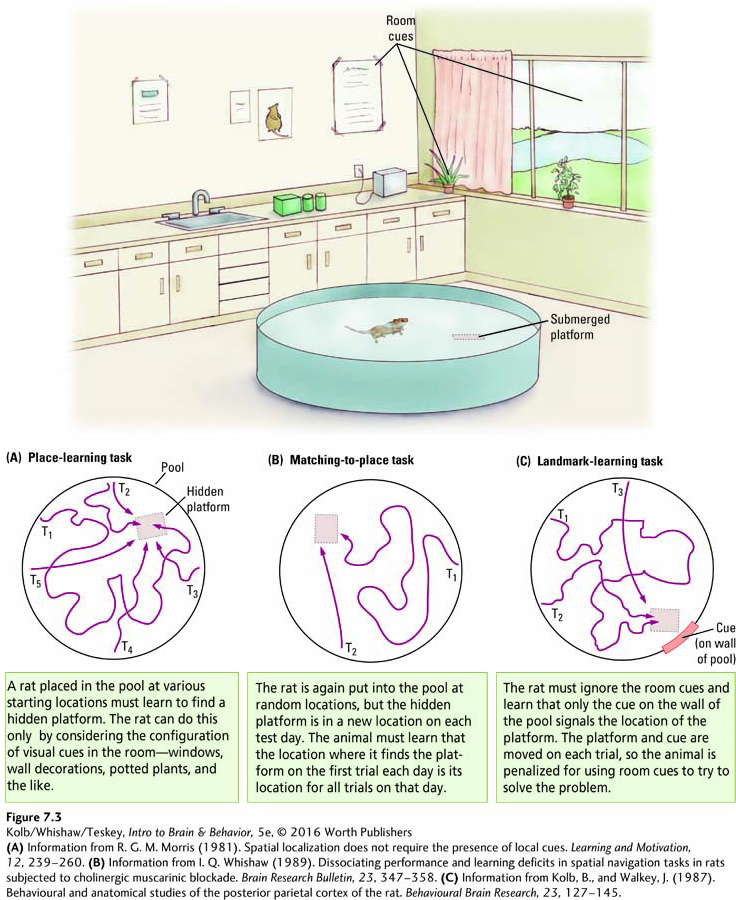
In one version of the task, place learning, the rat must find the platform from any starting location in the pool (Figure 7-3A). The only cues available are outside the pool, so the rat must learn the relation between several cues in the room and the platform’s location. In a second version of the task, matching-
The challenge for the rat in the matching-
Another type of behavioral analysis in rats is related to movement. A major problem facing people with stroke is a deficit in controlling hand and limb movements. The prevalence of stroke has prompted considerable interest in devising ways to analyze such motor behaviors for the purpose of testing new therapies for facilitating recovery. Ian Whishaw (Whishaw & Kolb, 2005) has devised both novel tasks and novel scoring methods to measure the fine details of skilled reaching movements in rats.
Experiments in Chapter 14 demonstrate fear conditioning in rats, plasticity in the monkey’s motor cortex, and neuronal effects of amphetamine sensitization in rats.
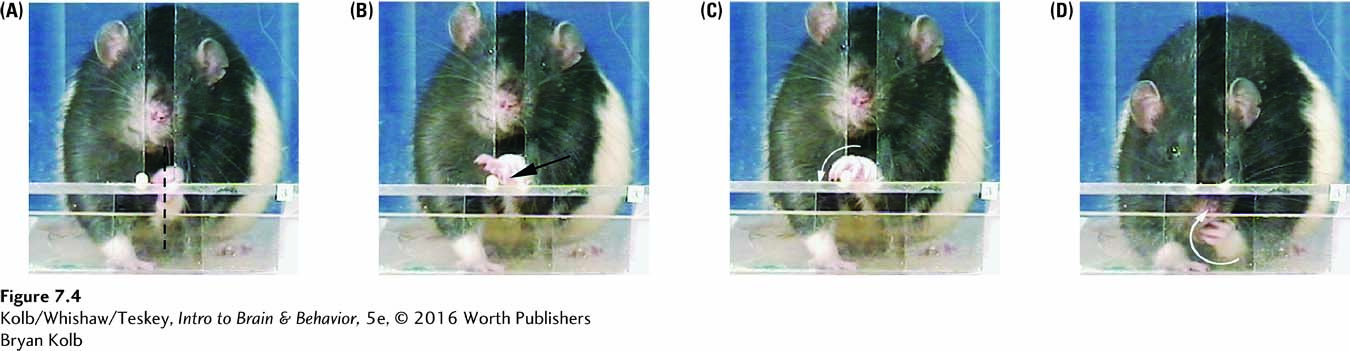
In one test, rats are trained to reach through a slot to obtain a piece of sweet food. The movements, which are remarkably similar to the movements people make in a similar task, can be broken down into segments. Investigators can score the segments separately, as they are differentially affected by different types of neurological perturbation.
The photo series in Figure 7-4 details how a rat orients its body to the slot (A), puts its hand through the slot (B), rotates the hand horizontally to grasp the food (C), then rotates the hand vertically and withdraws it to obtain the food (D). Contrary to reports common in neurology textbooks, primates are not the only animals to make fine digit movements, but because the rat’s hand is small and moves so quickly, digit dexterity can be seen in rodents only with use of high-
Manipulating Brain–Behavior Interactions
One strategy for studying brain–
Focus features 5-2, 5-3, and 5-4 detail many aspects of Parkinson disease.
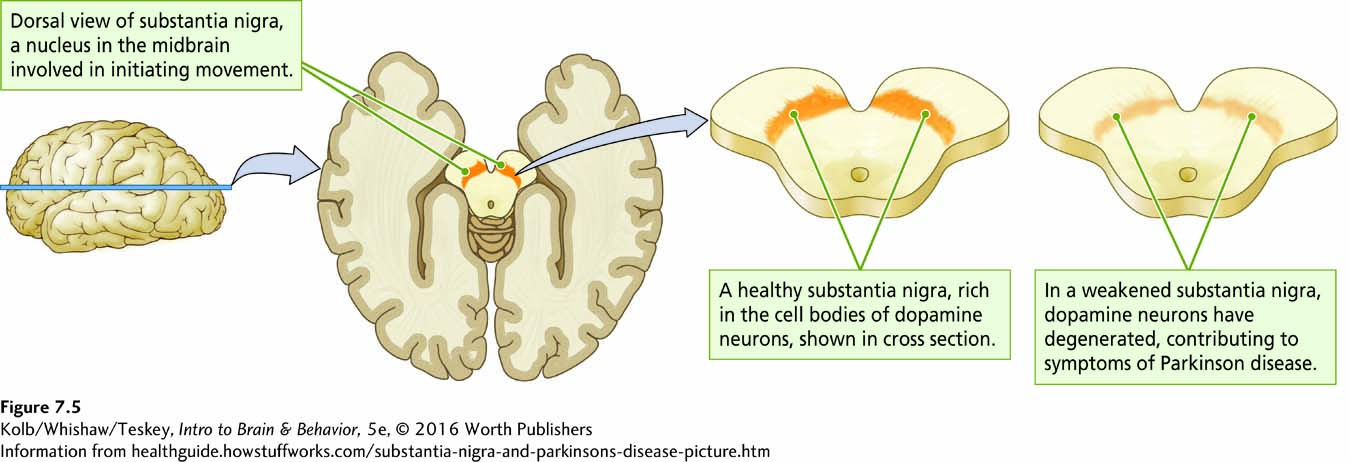
Neuroscientists hypothesize about the functions of brain regions by studying how removing or inhibiting them affects behavior. Broca studied patients with naturally occurring injuries and made inferences about language organization. Similarly, Parkinson patients have motor disturbances and associated cell death in the substantia nigra (Figure 7-5). It is a small step experimentally to injure specific brain regions in laboratory animals, then study their behavior. Such studies tell investigators not only about the injured region’s function but also what the remaining brain can do in the absence of the injured region. Neuroscientists also study brain region functions to form hypotheses, for example, on how exciting a region affects behavior. Certain drugs and stimulation techniques that increase brain region activity result in behavioral change.
A second reason to manipulate the brain is to develop animal models of neurological and psychiatric disorders. The general presumption in neurology and psychiatry is that it ought to be possible to restore at least some healthy functioning by pharmacological, behavioral, or other interventions. A major hurdle for developing such treatments is that, like most new medical treatments, they must be tested in nonhuman subjects first. (In Section 7-7, we take up scientific and ethical issues surrounding the use of animals in research.)
For brain disorders, researchers must develop models of diseases before they can be treated. Consider dementia, a progressive memory impairment that appears to be related to neuronal death in specific brain regions. The goal for treatment is to reverse or prevent cell death, but developing effective treatments requires an animal model that mimics dementia.
Brains can be manipulated in various ways, the precise manner depending on the specific research question being asked. Researchers can manipulate the whole animal by exposing it to differing diets, social interactions, exercise, sensory stimulation, and a host of other experiences. For brain manipulation, the principal direct techniques are to inactivate the brain via lesion or with drugs or to activate it with electrical stimulation, drugs, or light.
Brain Lesions
The first—
Scoville’s patient, H. M., profiled in Section 14-2, became the most-
To his chagrin, Lashley failed in his quest. He observed instead that memory loss was related to the amount of tissue he removed. The only conclusion Lashley could reach was that memory is distributed throughout the brain and not located in any single place. Subsequent research strongly indicates that specific brain functions and associated memories are indeed localized to specific brain regions. Ironically, just as Lashley was retiring, William Scoville and Brenda Milner (1957) described a patient from whose brain Scoville had removed both hippocampi as a treatment for epilepsy. The surgery rendered this patient amnesic. During his ablation research, Lashley had never removed the hippocampi because he had no reason to believe the structures had any role in memory. And because the hippocampus is not accessible on the brain’s surface, other techniques had to be developed before subcortical lesions could be used.
The solution to accessing subcortical regions is to use a stereotaxic apparatus, a device that permits a researcher or a neurosurgeon to target a specific part of the brain for ablation, as shown in Figure 7-6. The head is held in a fixed position, and because the location of brain structures is fixed in relation to the junction of the skull bones, it is possible to visualize a three-
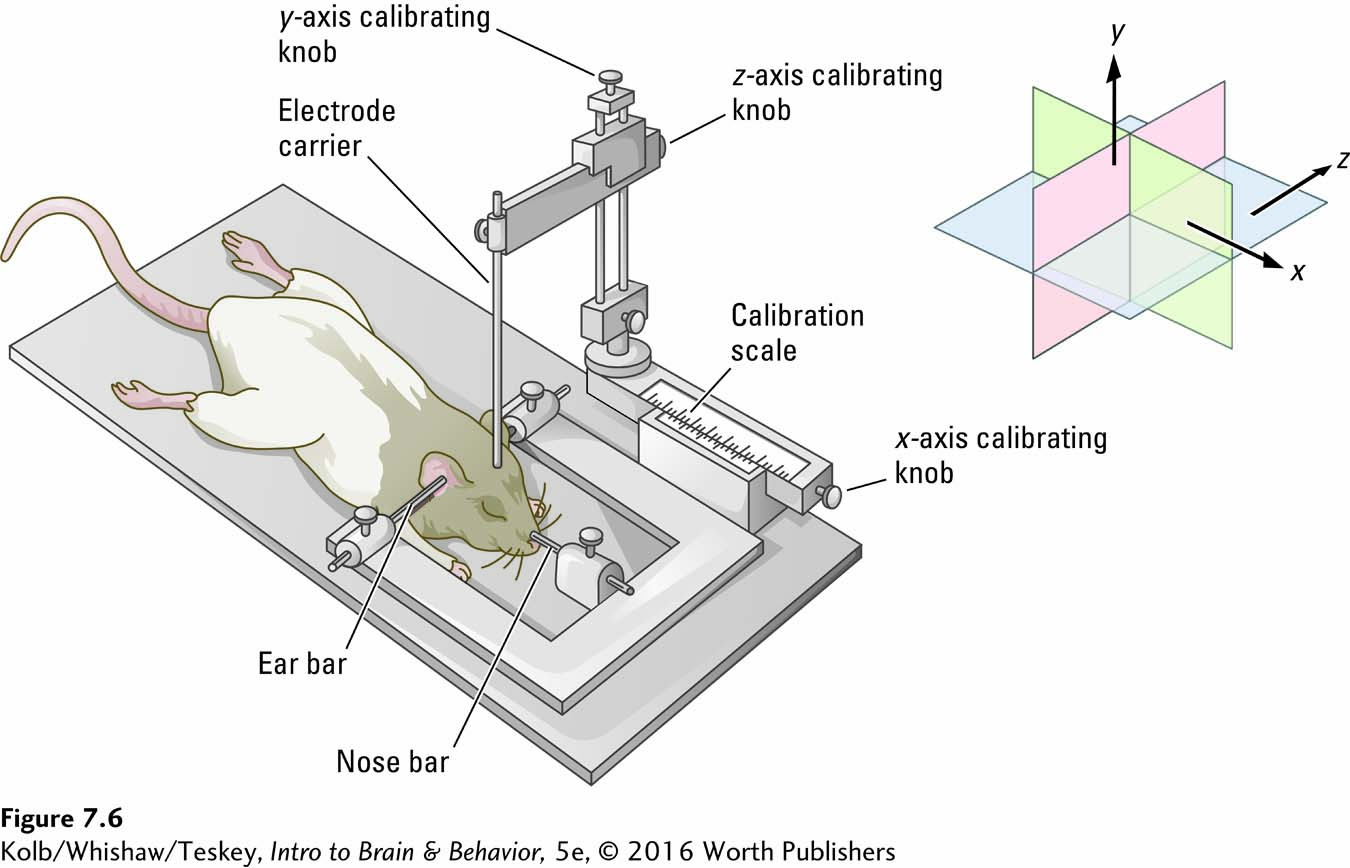
Review the brain’s anatomical locations and orientations in The Basics, in Section 2-1. The brain atlas in Figure 8-14 tracks cortical thickness over time.
Rostral–
Consider the substantia nigra. To ablate this region to induce a rat to display symptoms of Parkinson disease, the structure and its three-
A problem with electrolytic lesions: not only are the neurons of the targeted tissue killed but so are any nerve fibers passing through the region (in this case, substantia nigra). One solution is to lower a narrow metal tube (a cannula) instead of an electrode, infuse a neuron-

To make a rat parkinsonian, a toxin can be injected that is selectively taken up by dopaminergic neurons; this leads to a condition that mimics human Parkinson pathology. Animals with such neurotoxic lesions have a variety of motor symptoms including hypokinesia (slowness or absence of movement), short footsteps, and tremor. Drugs such as L-dopa, an agonist that enhances dopamine production, and atropine, an antagonist that blocks acetylcholine production, relieve these symptoms in Parkinson patients. Ian Whishaw and his colleagues (Schallert et al., 1978) thus were able to selectively lesion the substantia nigra in rats to produce a behavioral model of Parkinson disease.
The invasive techniques described so far result in permanent brain damage. With time, the research subject will show compensation, the neuroplastic ability to modify behavior from that used prior to the damage. To avoid compensation following permanent lesions, researchers have also developed temporary and reversible lesion techniques such as regional cooling, which prevents synaptic transmission. A hollow metal coil is placed next to a neural structure; then chilled fluid is passed through the coil, cooling the brain structure to about 18ºC (Lomber & Payne, 1996). When the chilled fluid is removed from the coil, the brain structure quickly warms, and synaptic transmission is restored. Another technique involves local administration of a GABA agonist, which increases local inhibition and in turn prevents the brain structure from communicating with other structures. Degradation of the GABA agonist reverses the local inhibition and restores function.
Brain Stimulation
Read more about Penfield’s dramatic discoveries in Sections 10-4 and 11-2.
The brain operates on both electrical and chemical energy, so it is possible to selectively turn brain regions on or off by using electrical or chemical stimulation. Wilder Penfield, in the mid-
Figure 12-12 diagrams hypothalamus anatomy. Section 12-3 details its role in motivated and emotional behavior.
Perhaps the most dramatic research example comes from stimulating specific regions of the hypothalamus. Rats with electrodes placed in the lateral hypothalamus will eat whenever the stimulation is turned on. If the animals have the opportunity to press a bar that briefly turns on the current, they quickly learn to press the bar to obtain the current, a behavior known as electrical self-
Brain stimulation can also be used as a therapy. When the intact cortex adjacent to cortex injured by a stroke is stimulated electrically, for example, it leads to improvement in motor behaviors such as those illustrated in Figure 7-4. Cam Teskey and his colleagues (Brown et al., 2011) successfully restored motor deficits in a rat model of Parkinson disease by electrically stimulating a specific brain nucleus.
View DBS in place in Figure 1-6.
Deep-
Electrical stimulation of the brain is invasive: holes must be drilled in the skull and an electrode lowered into the brain. Researchers have taken advantage of the relation between magnetism and electricity to develop a noninvasive technique, transcranial magnetic stimulation (TMS). A small wire coil is placed adjacent to the skull, as illustrated in Figure 7-7A. A high-
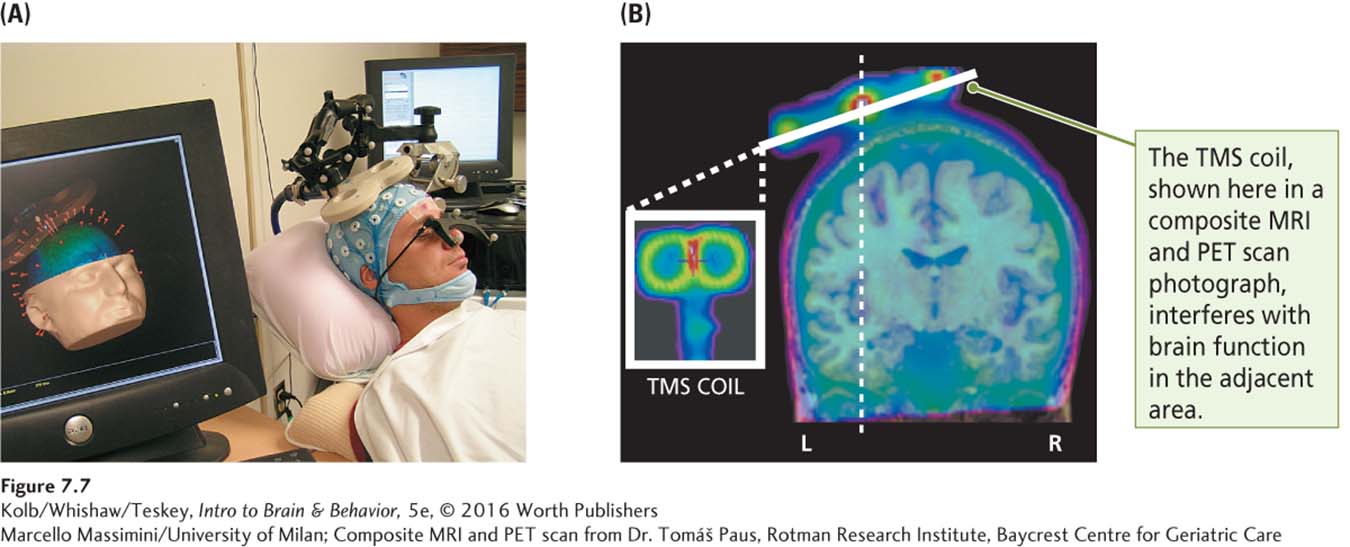
Composite MRI and PET scan from Dr. Tomáš Paus, Rotman Research Institute, Baycrest Centre for Geriatric Care
Research Focus 16-2 describes use of rTMS to treat depression and other behaviorral disorders.
If the motor cortex is stimulated, movement is evoked, or if a movement is in progress, it is disrupted. Similarly, if the visual cortex is stimulated, the participant sees dots of light (phosphenes). The effects of brief pulses of TMS do not outlive the stimulation, but repetitive TMS (rTMS), or continuous stimulation for up to several minutes, produces more long-
Drug Manipulations
Brain activity can also be stimulated by administration of drugs that pass into the bloodstream and eventually enter the brain or, through an indwelling cannula (illustrated in Figure 7-22), that allows direct application of them to specific brain structures. Drugs can influence the activity of specific neurons in specific brain regions. For example, the drug haloperidol, used to treat schizophrenia, reduces dopaminergic neuron function and makes healthy rats dopey and inactive (hypokinetic).
In contrast, drugs that increase dopaminergic activity, such as amphetamine, produce hyperkinetic rats—rats that are hyperactive. The advantage of administering drugs is that their effects wear off in time as the drugs are metabolized. It thus is possible to study drug effects on learned behaviors, such as skilled reaching (see Figure 7-4), and then to reexamine the behavior after the drug effect wears off.
Claudia Gonzalez and her colleagues (2006) administered nicotine to rats as they learned a skilled reaching task, then studied their later acquisition of a new skilled reaching task. The researchers found that the earlier nicotine-
Optogenetics and Chemogenetics
Within the span of a decade, synthetic biology, the design and construction of biological devices, systems, and machines not found in nature, has transformed how neuroscientists manipulate brain cells. Techniques include inserting a genetic sequence into the genome of a living organism. The transgenic technique of optogenetics, for example, combines genetics and light to control targeted cells in living tissue. A sequence that codes for a light-
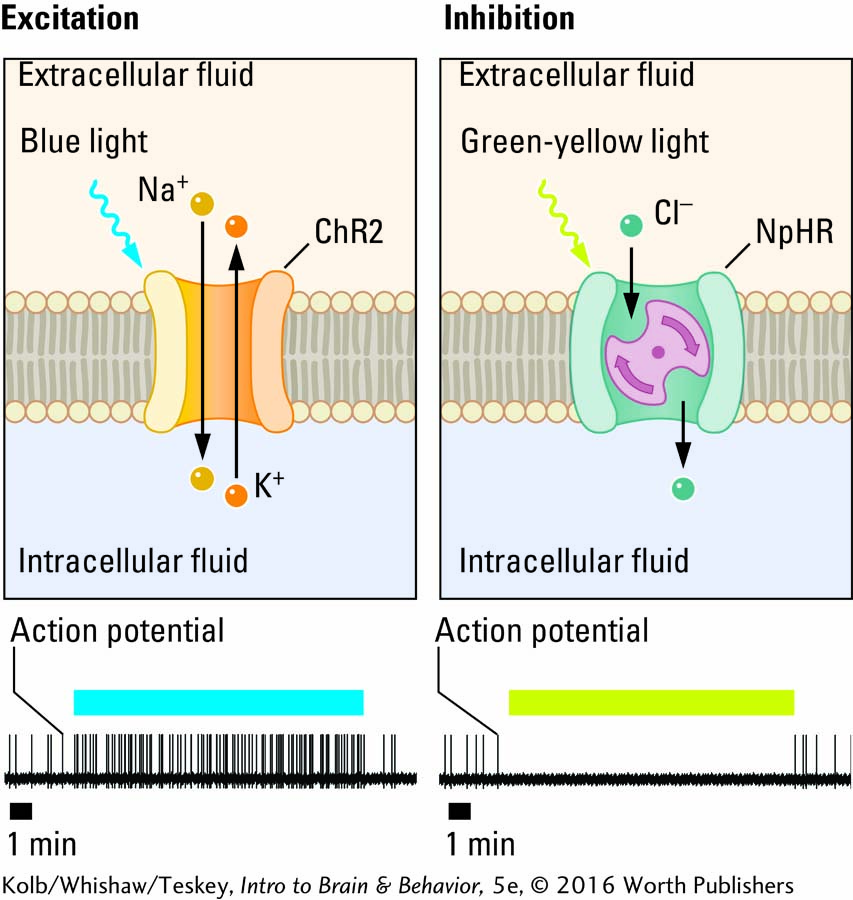
Optogenetics is based on the discovery that light can activate certain proteins that occur naturally and have been inserted into cells of model organisms. For example, opsins, proteins derived from microorganisms, combine a light-
Figure 2-25 diagrams the limbic structures.
Optogenetics has tremendous potential as a research tool. Investigators can insert light-
DREADD does not employ the sort of designer drug that may come to mind. Rather than synthetic versions of controlled substances (e.g., heroin), these synthetic drugs interact with a synthetic receptor.
In the transgenic technique, chemogenetics, the inserted synthetic genetic sequence codes for a G protein–
7-1 REVIEW
Measuring and Manipulating Brain and Behavior
Before you continue, check your understanding.
Question 1
Behavioral neuroscience, the study of relations between ________ and ________, employs memory tests such as ________ in nonhuman animals such as rats.
Question 2
Anatomical studies rely on techniques such as ________ tissue postmortem or visualizing living tissue using a ________.
Question 3
Methods developed to manipulate the brain include ________, ________, ________, and ________.
Question 4
Outline the various brain stimulation methods that either activate or inhibit neural activity.
Answers appear in the Self Test section of the book.