14-3 Neural Systems Underlying Explicit and Implicit Memories
Findings from laboratory studies, largely on rats and monkeys, have reproduced the symptoms of patients such as H. M. and J. K. by injuring the animals’ medial temporal regions and basal ganglia, respectively. Other structures, most notably in the frontal and temporal lobes, also participate in certain types of explicit memory. We now consider the systems for explicit and implicit memory separately.
Neural Circuit for Explicit Memories
Turns out, the hippocampus participates in species-
The dramatic amnesic syndrome discovered in H. M. in the 1950s led investigators to focus on the hippocampus, at the time regarded as a large brain structure in search of a function. But H. M. had other damaged structures, too, and the initial focus on the hippocampus as the location of explicit memory processing turned out to be misguided.
Consult sources published before 1995, and you may find explanations for memory far different from this chapter’s (see Gazzaniga, 2000).
After decades of anatomical and behavioral studies sorted out the complexities, consensus on the anatomy of explicit memory had coalesced by the mid-
Before considering the model, we first revisit medial temporal anatomy. Review Figure 14-5’s summary of findings, which show that memories for objects’ colors and motion characteristics reside in separate temporal lobe locations. Thus the medial temporal region receives multiple sensory inputs.
In Greek, para means beside; rhino means nose. The perirhinal cortex lies beside the rhinal sulcus on the bottom of the brain.
The macaque monkey’s medial temporal region shares many anatomical similarities with the region in the human brain. In addition to the hippocampus and amygdala, three medial temporal areas illustrated in Figure 14-8 take part in explicit memory: lying adjacentto the hippocampus are the entorhinal cortex, the parahippocampal cortex, and the perirhinal cortex. A sequential arrangement of two-
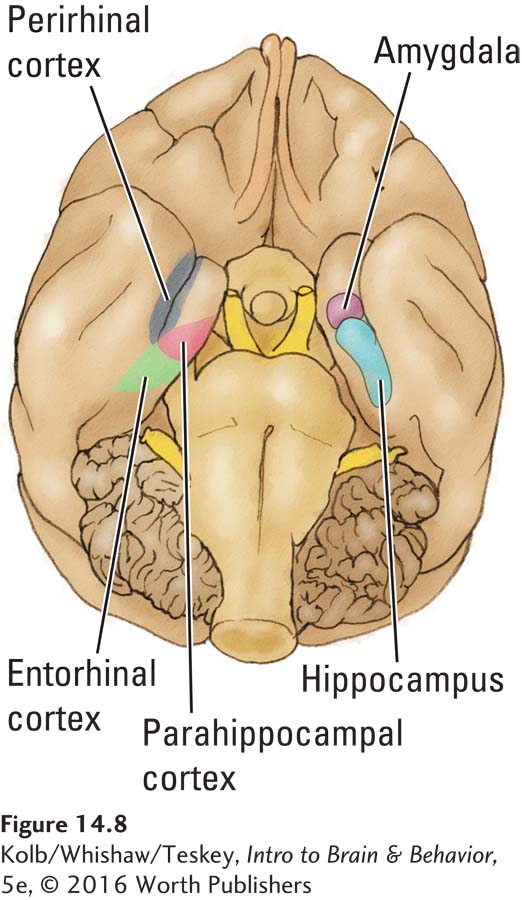
Section 9-2 traces visual pathways in detail.
The prominent cortical input to the perirhinal region is from the visual ventral stream coursing through the temporal lobe. The perirhinal region is thus a prime candidate location for visual object memory. Similarly, the parahippocampal cortex receives strong input from parietal regions believed to take part in visuospatial processing and likely participates in visuospatial memory—using visual information to recall an object’s location in space.
14-3
Alzheimer Disease
In the 1880s it was noted that the brain may undergo atrophy with aging, but the reason was not really understood until the German physician Alois Alzheimer published a landmark study in 1906. Alzheimer described a set of behavioral symptoms and associated neuropathology in a 51-
An estimated 5.4 million people in the United States have Alzheimer disease, although the only certain diagnostic test remains postmortem examination of cerebral tissue. The disease progresses slowly, and many people with Alzheimer disease probably die of other causes before the cognitive symptoms incapacitate them.
We knew of a physics professor who continued to work until, when he was nearly 80, he died of a heart attack. Postmortem examination of his brain revealed significant Alzheimer pathology. His colleagues had attributed the professor’s slipping memory to the old-
The cause of Alzheimer disease remains unknown, although it has been variously attributed to genetic predisposition, abnormal levels of trace elements (e.g., aluminum), immune reactions, slow viruses, and prions (abnormal, infectious forms of proteins). Two principal neuronal changes take place in Alzheimer disease:
- Loss of cholinergic cells in the basal forebrain. One treatment for Alzheimer disease, therefore, is medication that increases acetylcholine levels in the forebrain. An example is Exelon, which is the trade name for rivastigmine, a cholinergic agonist that appears to provide temporary relief from the progression of the disease and is available both orally and as a skin patch.
- Development of neuritic plaques in the cerebral cortex. A neuritic plaque consists of a central core of homogeneous protein material (amyloid) surrounded by degenerative cellular fragments. The plaques are not distributed evenly throughout the cortex but are concentrated especially in temporal lobe areas related to memory. Neuritic plaques are often associated with another abnormality, neurofibrillary tangles, paired helical filaments found in both the cerebral cortex and the hippocampus. The prion paradigm holds that misfolded tau proteins, illustrated here, which have the ability to self-
propagate, cause many age- related neurodegenerative diseases (Walker & Jucker, 2015). Researchers are conducting clinical trials on new drugs that act either to find and neutralize misfolded proteins or as immunizing agents, to prevent protein misfolding (Wisniewski & Goni, 2015).
Cortical neurons begin to deteriorate as the cholinergic loss, plaques, and tangles develop. The first cells to die are in the entorhinal cortex (see Figure 14-8). Significant memory disturbance ensues.
A controversial idea emerging from stroke neurologists is that dementia may reflect a chronic cerebrovascular condition, marginal high blood pressure. Marginal elevations in blood pressure can lead to cerebral microbleeds, especially in white matter. The cumulative effect of years or even decades of tiny bleeds would eventually lead to increasingly disturbed cognition. This may first appear as mild cognitive impairment (MCI) that slowly progresses with cumulative microbleeds.
Section 16-3 elaborates on Alzheimer and other dementias and on prion theory.
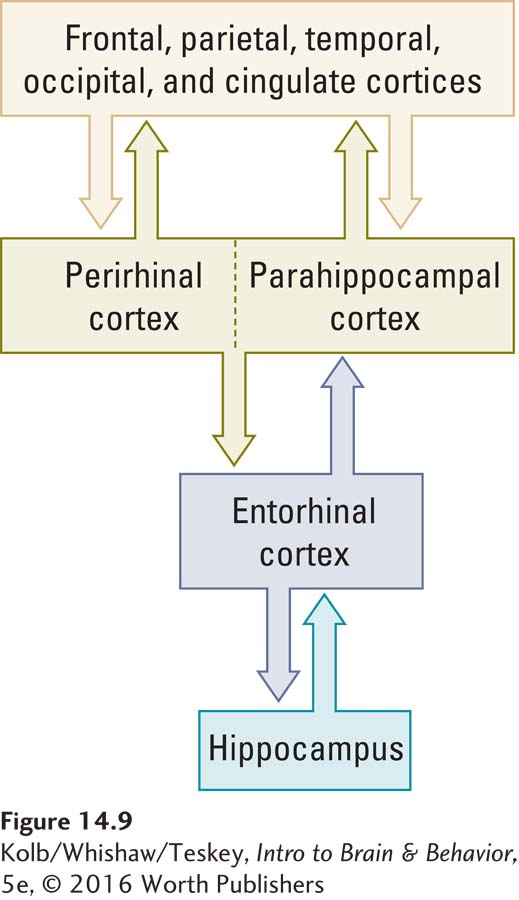
Because both the perirhinal and the parahippocampal regions project to the entorhinal cortex, this region probably participates in more integrative memory functions. The entorhinal cortex is in fact the first area to show cell death in Alzheimer disease, a dementia characterized by severe deficits in explicit memory (see Clinical Focus 14-3, Alzheimer Disease).
The Hippocampus and Spatial Memory
We are left with a conundrum. If the hippocampus is not the key structure in explicit memory even as it receives the entorhinal connections, what does it do? John O’Keefe and Lynn Nadel were the first to advance the idea, in 1978, that the hippocampus is probably engaged in visuospatial memory processes required for places, such as recalling an object’s location.
Certainly both laboratory animals and human patients with selective hippocampal injury have severe deficits in various forms of spatial memory. Similarly, monkeys with hippocampal lesions have difficulty learning the location of objects (visuospatial learning), as can be demonstrated in tasks such as the ones illustrated in Figure 14-10.
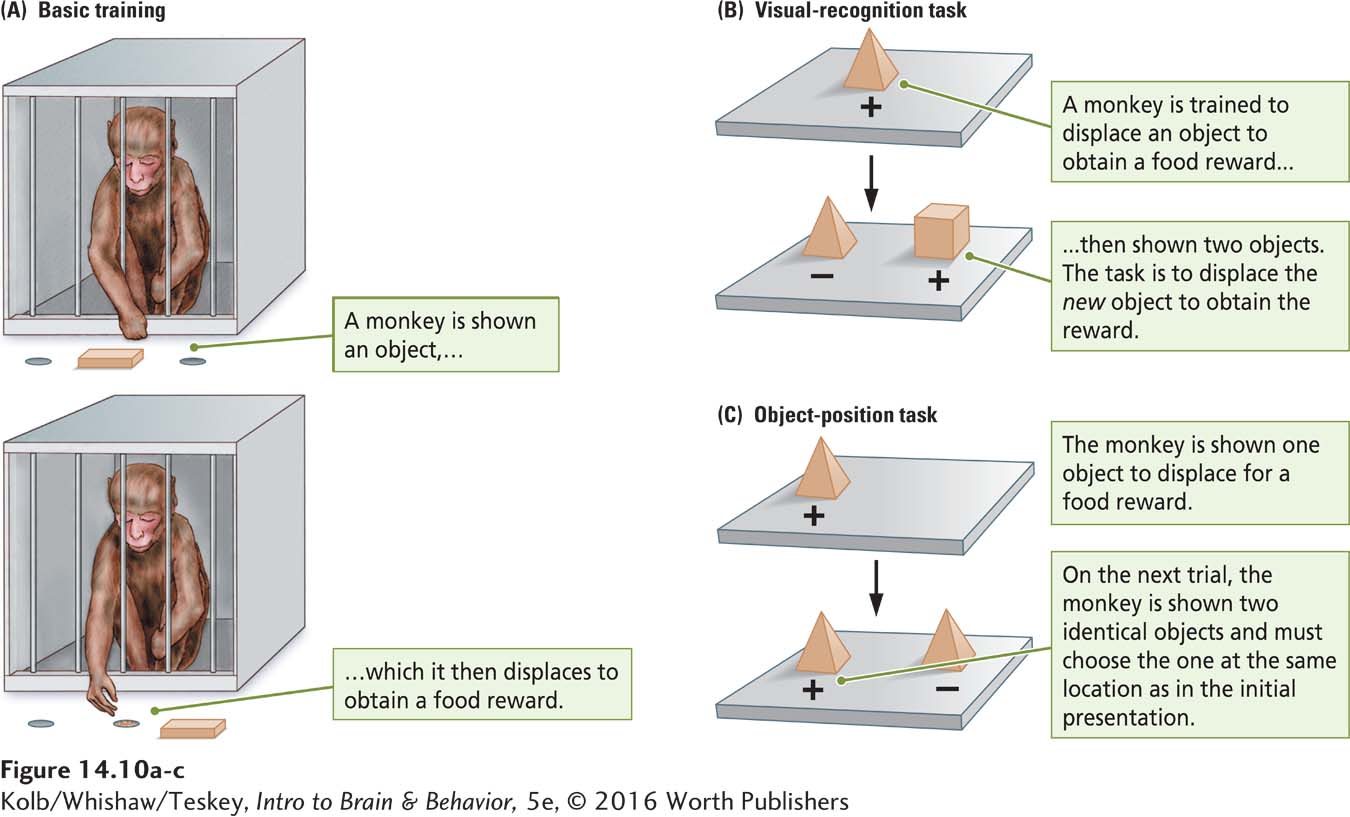
Monkeys are trained to displace objects to obtain a food reward (Figure 14-10A), then given one of two tasks. In the visual recognition task (Figure 14-10B), the animal displaces a sample object for the food reward. After a short delay, the animal is presented with two objects. One is novel. The task is to learn to displace the novel object for the food reward. This task tests explicit visual object memory. Monkeys with perirhinal lesions are impaired at the task.
In the object position task in Figure 14-10C, the monkey is shown one object to be displaced for a food reward. Then the monkey is shown the same object along with a second identical one. The task is to learn to displace the object that is in the same position as it was in the initial presentation. Monkeys with hippocampal lesions are selectively impaired at this task.
Section 1-4 explains the encephalization quotient, an index of ratios of brain to body size. EQs allow comparisons of the relative brain sizes of different species.
From these results, we would predict that a species with an especially good spatial memory should have bigger hippocampi than do species with a poorer spatial memory. David Sherry and his colleagues (1992) tested this hypothesis in birds.
Many birds are cachers: they harvest sunflower seeds and other favored foods and hide (cache) them to eat later. Some birds can find hundreds of items they have cached. To evaluate whether the hippocampus plays a role in this activity, Sherry and his coworkers measured hippocampal size in closely related bird species, only one of which is a food cacher. As shown in Figure 14-11, the hippocampal formation is larger in birds that cache food than in birds that do not. In fact, the hippocampi of food-
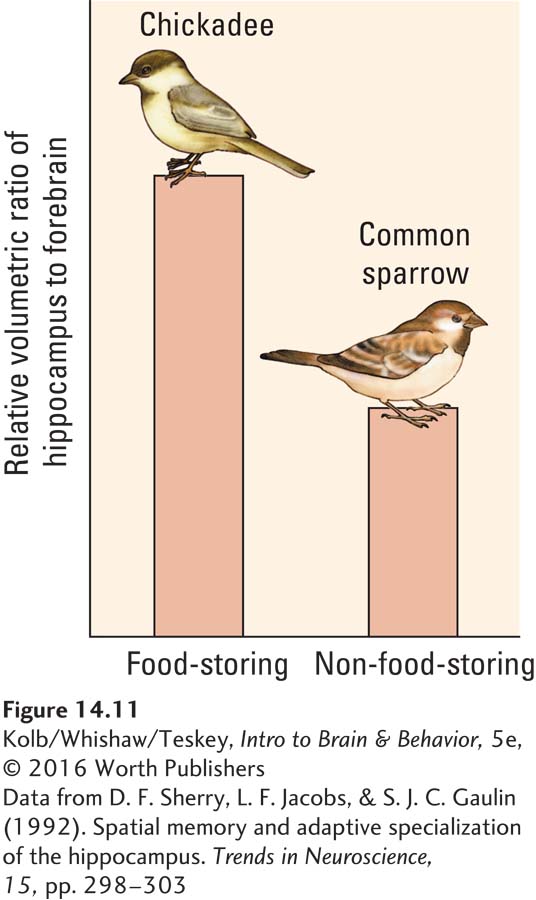
Sherry found a similar relation when he compared different species of food-
One prediction we might make based on the Sherry experiments is that people who have a job with high spatial demands have large hippocampi. Taxi drivers in London fit this category. Successful candidates for a cab driver’s license in London must demonstrate that they know the location of every street in that huge and ancient city. Using MRI, Eleanor Maguire and her colleagues (2000) found the posterior region of the hippocampus in London taxi drivers to be significantly larger than the same region in the control participants. This finding presumably explains why a select few pass a spatial memory test that most of us would fail miserably.
Spatial Cells in the Hippocampal Formation
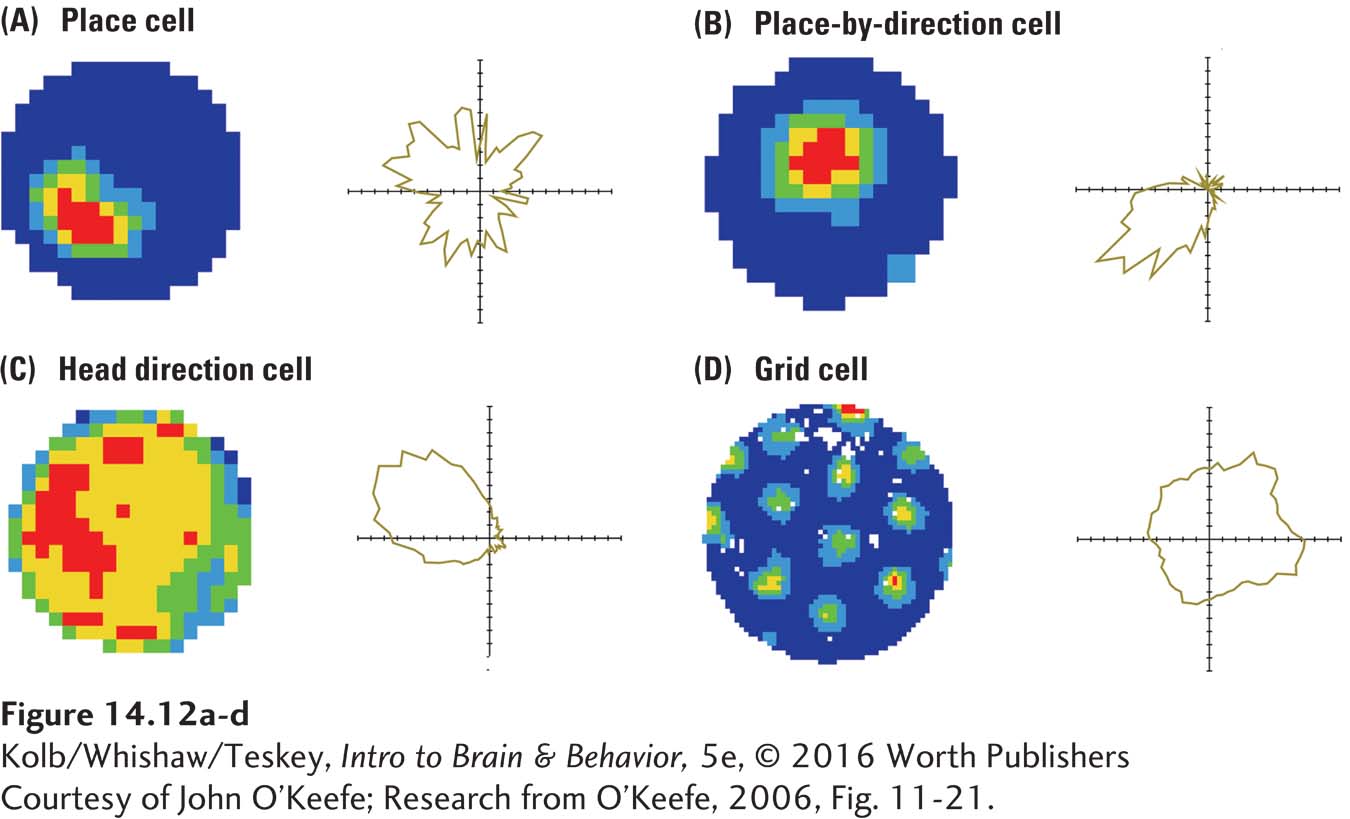
Given the role of the hippocampus in spatial behavior, we might predict that individual cells would code spatial information. They do. Three classes of spatially related cells have been identified in the rat and mouse hippocampus (Figure 14-12). Together they form an internal GPS, a neural global positioning system. That is, neurons vigorously fire when an animal is in a specific place in the environment. In 2014, John O’Keefe, Edvard Moser, and May-
Courtesy of John O’Keefe.
Place cells, shown in Figure 14-12A and B, discharge when rats are in a spatial location, irrespective of its orientation. Head direction cells (Figure 14-12C) discharge whenever a rat’s head points in a particular direction. Grid cells (Figure 14-12D) discharge at many locations, forming a virtual grid invariant to changes in the rat’s direction, movement, or speed.
These cells are not localized to the hippocampus but are found within a network that includes the hippocampus as its central structure. Place cells and head direction cells are located in the hippocampus and closely related structures. Grid cells are found in the entorhinal cortex, a major afferent route into the hippocampus (see Figure 14-9). Taken together, we can envision the place cell system as indicating where things are in the world, the grid system indicating how big our present navigating environment is, and the head direction and grid systems telling us where we ourselves are in the environment.
Reciprocal Connections for Explicit Memory
Explicit memory’s temporal pathway is reciprocal: connections from the neocortex run to the entorhinal cortex, then back to the neocortex (see Figure 14-9). Reciprocal connections have two benefits:
- Signals from the medial temporal regions back to the cortical sensory regions keep the sensory experience alive in the brain: the neural record of an experience outlasts the actual experience.
- The pathway back to the neocortex keeps it appraised of information being processed in the medial temporal regions.
The basal ganglia systems that take part in implicit memory do not feed back to the cortex, which helps explain its unconscious nature.
Although we have focused on the medial temporal regions, other structures also are important in explicit memory. People with frontal lobe injuries are not amnesic like H. M. or J. K., but they do have difficulties with memory for the temporal (time) order of events. Imagine being shown a series of photographs and asked to remember them. A few minutes later, you are asked whether you recognize two photographs and if so, to indicate which one you saw first.
H. M. would not remember the photographs. People with frontal lobe injuries would recall seeing the photographs but would have difficulty recalling which one they had seen more recently. The frontal lobe’s role in explicit memory clearly is subtler than that of the medial temporal lobe.
THE FRONTAL LOBE AND SHORT-
Joaquin Fuster (e.g., Fuster, Bodner, & Kroger, 2000) studied single-
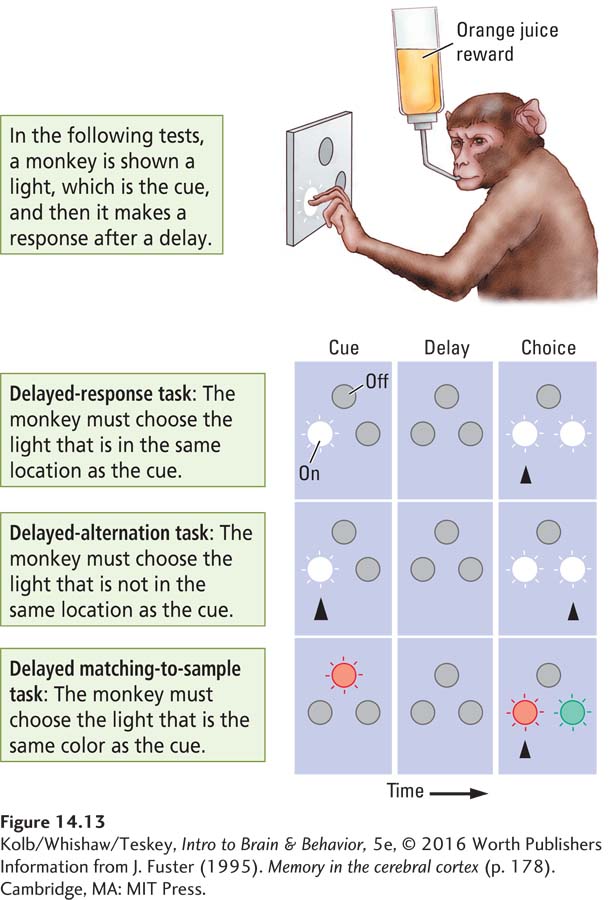
In the general design for each test, a monkey is shown a light (the cue), and after a delay it must make a response to get a reward.
In the delayed-
response task, the monkey is shown two lights in the choice test and must choose the one that is in the same location as the cue.In the delayed-
alternation task, the monkey is again shown two lights in the choice tests but now must choose the light that is not in the same location as the cue.In the delayed matching-
to- the monkey is shown, say, a red light, then, after a delay, a red and a green light. The task is to choose the red light regardless of its new location.sample task,
Fuster found that in each task certain cells in the prefrontal cortex fire throughout the delay. Animals that have not learned the task show no such cell activity. Curiously, if a trained animal makes an error, its cellular activity corresponds: the cells stop responding before the error occurs. They have “forgotten” the cue.
TRACING THE EXPLICIT MEMORY CIRCUIT People who have chronically abused alcohol can develop an explicit memory disturbance known as Korsakoff syndrome. In some cases, severe deficits in explicit memory extend to implicit memory as well. Korsakoff syndrome is caused by a thiamine (vitamin B1) deficiency that kills cells in the medial part of the diencephalon—
14-4
Korsakoff Syndrome
Over the long term, alcoholism, especially when accompanied by malnutrition, obliterates memory. When 62-
When asked what he had done the previous night, Joe R. typically said that he “went to the Legion for a few beers with the boys.” Although he had, in fact, been in the hospital, it was a sensible response because going to the Legion is what he had done on most nights in the preceding 30 years.
Joe R. was not certain what he had done for a living but believed he had been a butcher. In fact, he had been a truck driver for a local delivery firm. His son was a butcher, however, so once again his story related to something in his life.
Joe’s memory for immediate events was little better. On one occasion, we asked him to remember having met us; then we left the room. On our return 2 or 3 minutes later, he had no recollection of ever having met us or of having taken psychological tests that we had administered.
Joe R. had Korsakoff syndrome. Sergei Korsakoff was a Russian physician who in the 1880s first called attention to a syndrome that accompanies chronic alcoholism. The most obvious symptom is severe memory loss, including amnesia for both information learned in the past (retrograde amnesia) and information learned since the onset of the memory disturbance (anterograde amnesia).
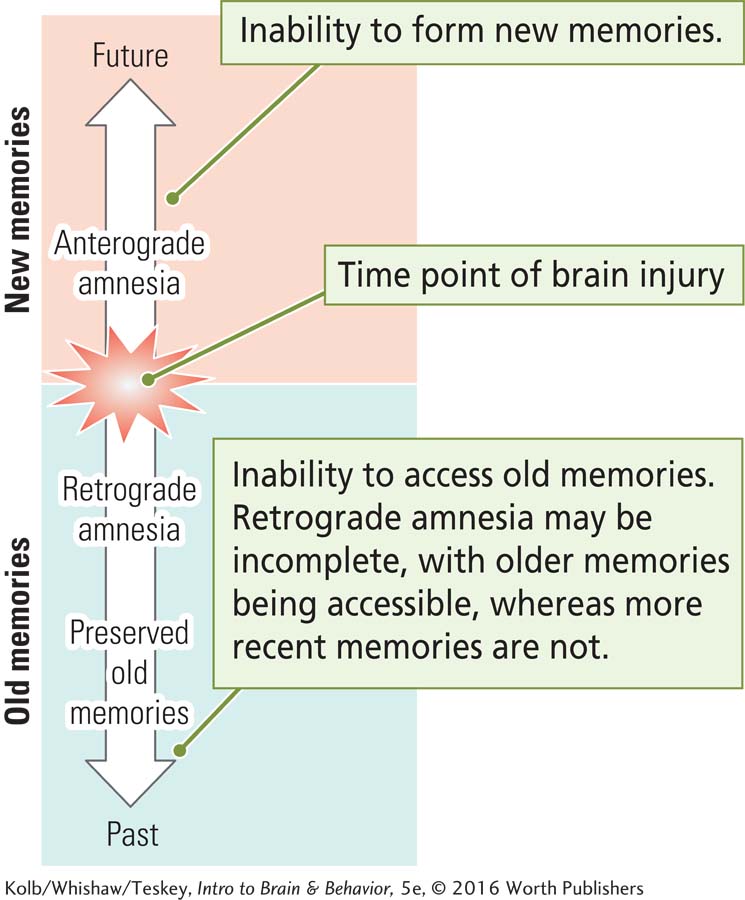
One unique characteristic of the amnesic syndrome in Korsakoff patients is that they tend to make up stories about past events rather than admit that they do not remember. These stories are generally plausible, like those Joe R. told, because they are based on actual experiences.
Curiously, Korsakoff patients have little insight into their memory disturbance and are generally indifferent to suggestions that they have a memory problem. Such patients are generally apathetic to what’s going on around them too. Joe R. was often seen watching television when the set was turned off.
The cause of Korsakoff syndrome is a thiamine (vitamin B1) deficiency resulting from poor diet and prolonged intake of large quantities of alcohol. (In addition to a “few beers with the boys,” Joe R. had a long history of drinking a 26-
Most Korsakoff patients also show cortical atrophy, especially in the frontal lobe. With the appearance of Korsakoff symptoms, which can happen suddenly, prognosis is poor. Only about 20 percent of patients show much recovery after a year on a vitamin B1–enriched diet. Joe R. has shown no recovery after several years and will spend the rest of his life in a hospital setting.
Mortimer Mishkin and his colleagues (Mishkin, 1982; Murray, 2000) proposed a neural circuit for explicit memory that incorporates the evidence from both humans and laboratory animals with injuries to the temporal and frontal lobes. Figure 14-14 presents a modified version of the Mishkin model. Anatomically (Figure 14-14A), it includes not only the frontal and temporal lobes but also the medial thalamus, implicated in Korsakoff syndrome, and the basal forebrain–
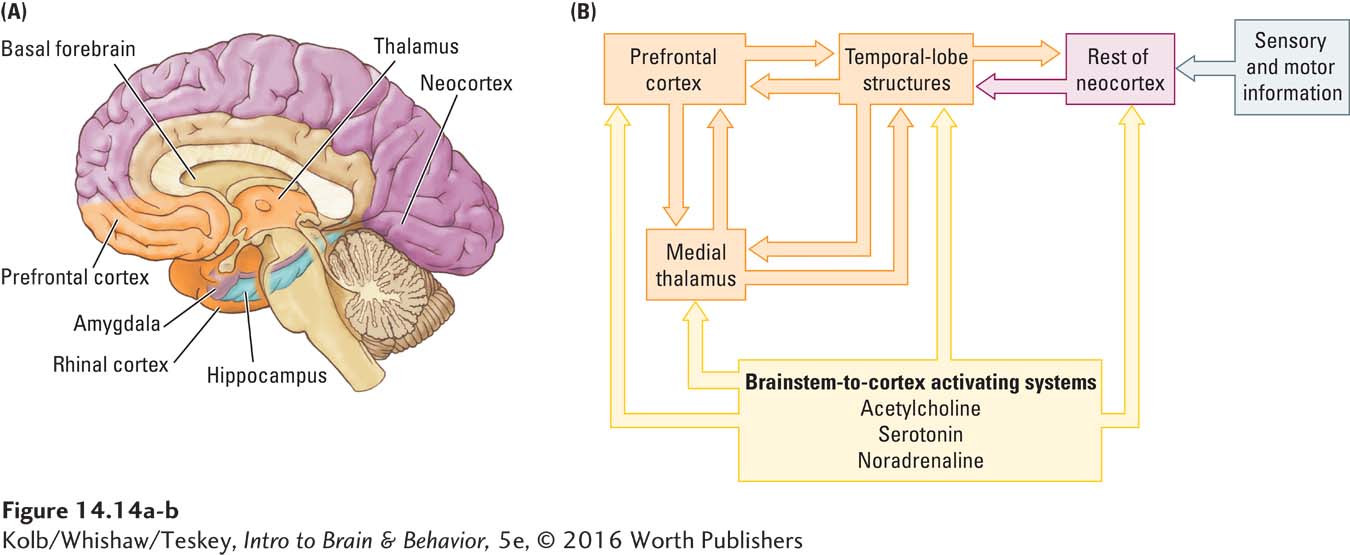
Sensory and motor neocortical areas send their connections to the medial temporal regions, which are in turn connected to the medial thalamus and prefrontal cortex.
Basal forebrain structures are hypothesized to play a role in maintaining appropriate activity levels in other forebrain structures so that they can process information.
The temporal lobe structures are hypothesized to be central to long-
term explicit memory formation. The prefrontal cortex is central to maintaining temporary (short-
term) explicit memories as well as memory for the recency (chronological order) of explicit events.
Consolidation of Explicit Memories
Amnesia often appears to be time-
This idea led to the hypothesis that the hippocampus consolidates new memories, a process that makes them permanent. In consolidation, or stabilizing a memory trace after learning, memories move from the hippocampus to diffuse neocortical regions. Once they move, hippocampal involvement is no longer needed.
It is not clear how memories are moved, however, or how long it takes. Robert Sutherland and his colleagues (2010) propose a model of consolidation, the distributed reinstatement theory. In their model, a learning episode rapidly produces a stored memory representation that is strong in the hippocampus but weak elsewhere. The memory is replayed on the time scale of hours or days after the learning, leading to enhanced representations outside the hippocampus. Each repetition of the learning—
Memory is not constant over time. When people get misleading information about events they have experienced, for example, their later recall of those events often is modified. Indeed, this contributes to the notorious unreliability of eyewitness testimony. (Do you remember the “smashing” and “bumping” car collisions from Section 14-1?) The fact that memories appear changeable seems to fly in the face of the consolidation concept, which presumes that once consolidated, memories are fixed.
One solution to this conundrum suggests that whenever a memory is replayed in the mind, it is open to further consolidation, or reconsolidation, the process of restabilizing a memory trace after the memory is revisited. Interest in this idea has been intense, and although the final story is yet to be written, it appears that reconsolidation does occur, at least for some types of memories.
One way to think of the process is to see memory consolidation as never-
Section 5-4 links sensitization to PTSD. Sections 6-5 and 12-4 document how stress fosters and prolongs its effects, and Focus 16-1 covers treatments.
One implication of reconsolidation is that it ought to be possible to erase negative memories by using amnesic agents when the memory is revisited. This idea has important implications for reducing or eliminating the effects of strong emotional experiences, such as those seen in posttraumatic stress disorder (PTSD). One promising agent is the inert gas xenon, which has been used in humans as a fast-
Neural Circuit for Implicit Memories
Hypothesizing that the basal ganglia are central to implicit memory, Mishkin and his colleagues proposed a neural circuit for implicit memories (Mishkin, 1982; Mishkin et al., 1997). As Figure 14-15 shows, the basal ganglia receive input from the entire neocortex and send projections first to the ventral thalamus and then to the premotor cortex. The basal ganglia also receive widely and densely distributed projections from dopamine-
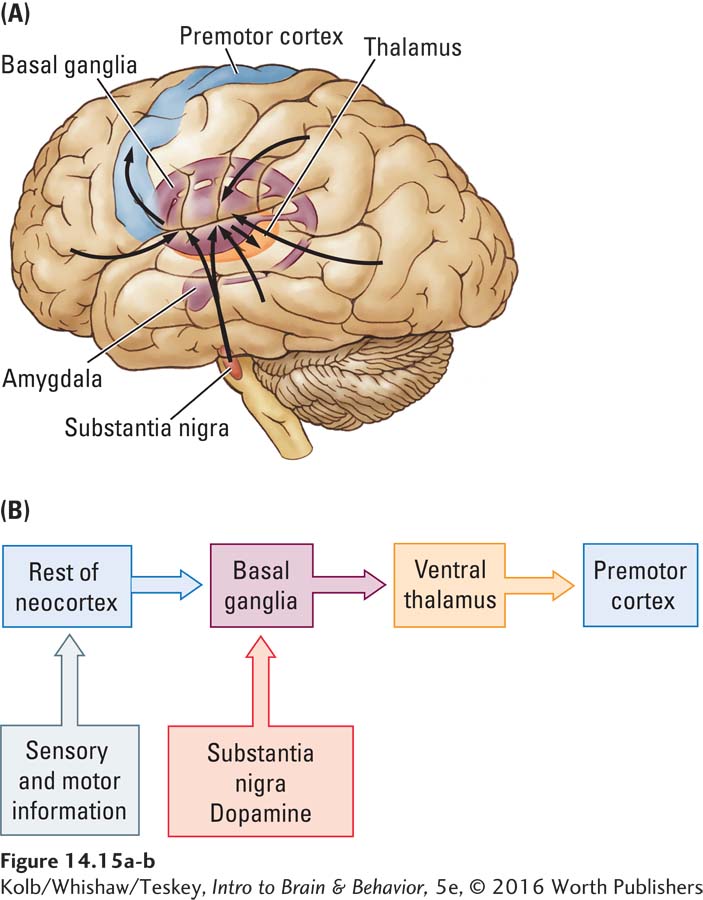
The connection from the cortex to the basal ganglia in the implicit memory system flows only in one direction. Most of the neocortex receives no direct information regarding activities in the basal ganglia. Mishkin believes that this unidirectional flow accounts for the unconscious nature of implicit memories. For memories to be conscious, the neocortical regions involved must receive feedback, as they do in the explicit memory system Mishkin and colleagues proposed (see Figure 14-14).
Mishkin’s models show why people with basal ganglia dysfunction, as occurs in Parkinson disease, have implicit memory deficits. People with frontal or temporal lobe injuries, by contrast, have relatively good implicit memories even as they may have profound explicit memory disturbances. Some people with Alzheimer disease can play games expertly, even with no recollection of having played them before.
Daniel Schacter (1983) wrote of a golfer with Alzheimer disease whose medial temporal system was severely compromised by the disease, but his basal ganglia were unaffected. Despite his explicit knowledge impairment, as indexed by his inability to find balls he had shot or to remember how many strokes he made on each hole, the man retained his ability to play the game.
Neural Circuit for Emotional Memories
Whether emotional memory for the affective properties of stimuli or events is implicit or explicit is not altogether clear. It could be both. Certainly people can react with fear to specific stimuli they can identify, and we have seen that they can also fear situations for which they do not seem to have specific memories. Panic disorder is a common pathology of emotional memory. People show marked anxiety but cannot identify a specific cause. Emotional memory has a unique anatomical component—
Section 12-4 details the amygdala’s influence on emotional behavior. Focus 12-3 describes panic and other anxiety disorders.
Emotional memory has been studied most thoroughly in fear conditioning by pairing unpleasant stimuli, such as foot shock, with a tone. Michael Davis (1992) and Joseph LeDoux (1995) used fear conditioning to demonstrate that the amygdala is critical to emotional memory. Damage to the amygdala abolishes emotional memory but has little effect on implicit or explicit memory.
The amygdala has close connections with medial temporal cortical structures as well as with the rest of the cortex. It also sends projections to brainstem structures that control autonomic responses such as blood pressure and heart rate; to the hypothalamus, which controls hormonal systems; to the periaqueductal gray matter (PAG), which affects pain perception; and to the enteric nervous system. The amygdala hooks in to the implicit memory system through its connections with the basal ganglia (Figure 14-16).
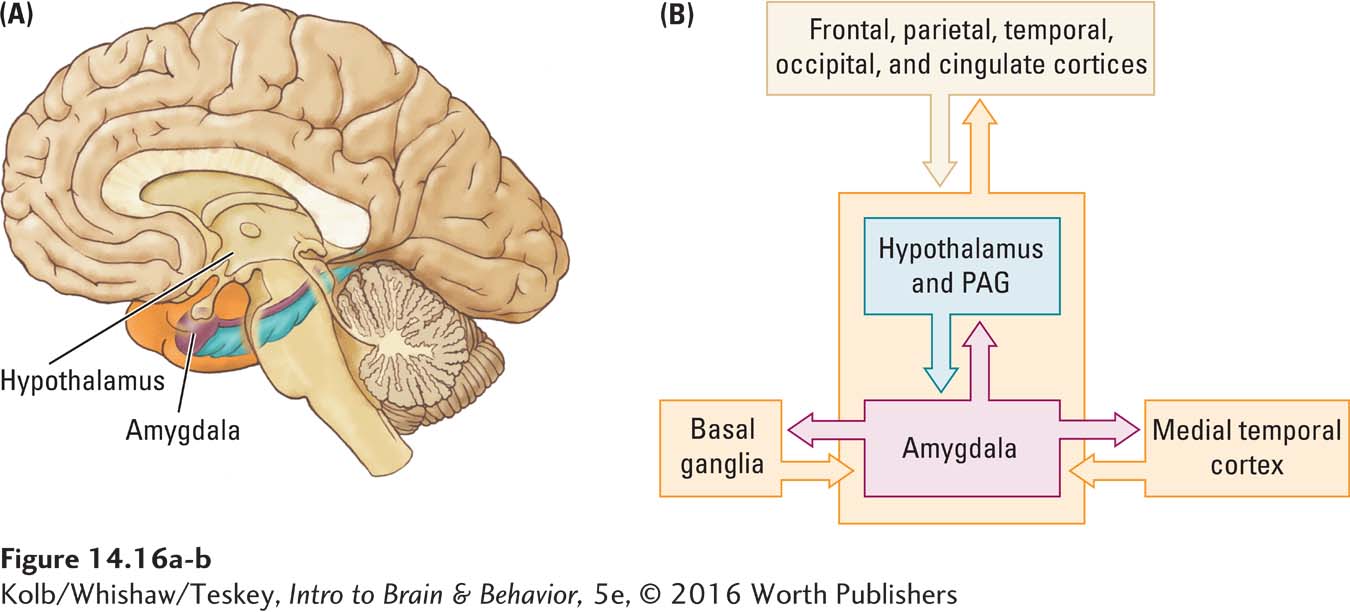
The ANS monitors and controls life support functions (Figure 2-
Fear is not the only aspect of emotional memory the amygdala codes, as a study of severely demented patients by Bob Sainsbury and Marjorie Coristine (1986) nicely illustrates. The patients were believed to have severe cortical abnormalities but intact amygdalar functioning. The researchers first established that the patients’ ability to recognize photographs of close relatives was severely impaired.
The patients were then shown four photographs, one depicting a relative (either a sibling or a child) who had visited in the past 2 weeks. The task was to identify the person whom they liked better than the other three. Although the subjects were unaware they knew anyone depicted in the photographs, they consistently preferred pictures of their relatives. This result suggests that although the explicit, and probably the implicit, memory of the relative was gone, each patient‘s emotional memory guided his or her preference.
We tend to remember emotionally arousing experiences vividly, a fact confirmed by findings from both animal and human studies. James McGaugh (2004) concluded that emotionally significant experiences, pleasant and unpleasant, must activate hormonal and brain systems that act to stamp in these vivid memories.
Figure 5-17 traces neural activating system connections. Section 6-5 explains how hormones work.
McGaugh noted that many neural systems probably take part, but the basolateral part of the amygdala is critical. The general idea is that emotionally driven hormonal and neurochemical activating systems (probably cholinergic and noradrenergic) stimulate the amygdala. The amygdala in turn modulates the laying down of emotional memory circuits in the rest of the brain, especially in the medial temporal and prefrontal regions and in the basal ganglia. We would not expect people with amygdala damage to have enhanced memory for emotion-
14-3 REVIEW
Neural Systems Underlying Explicit and Implicit Memories
Before you continue, check your understanding.
Question 1
The two key structures for explicit memory are ____________ and ____________.
Question 2
A system consisting of the basal ganglia and neocortex forms the neural basis of the ____________ memory system.
Question 3
The ____________ and associated structures form the neural basis for emotional memory.
Question 4
The progressive stabilization of memories is known as ____________.
Question 5
Why do we remember emotionally arousing experiences so vividly?
Answers appear in the Self Test section of the book.