8-2 Neurobiology of Development
Some 2000 years ago, the Roman philosopher Seneca the Younger proposed that a human embryo is an adult in miniature, and thus the task of development is simply to grow bigger. This idea of preformation was so appealing that it was widely believed for centuries. Even with the development of the microscope, the appeal of preformation proved so strong that biologists claimed to see microscopic horses in horse semen.
By the mid-
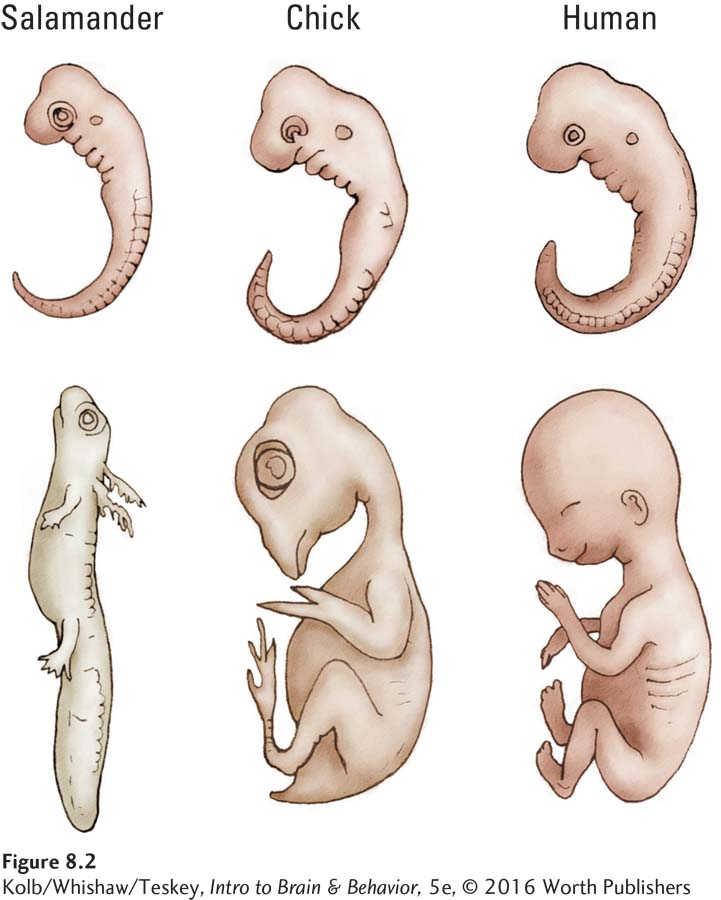
As embryos, all vertebrate species have a similar-
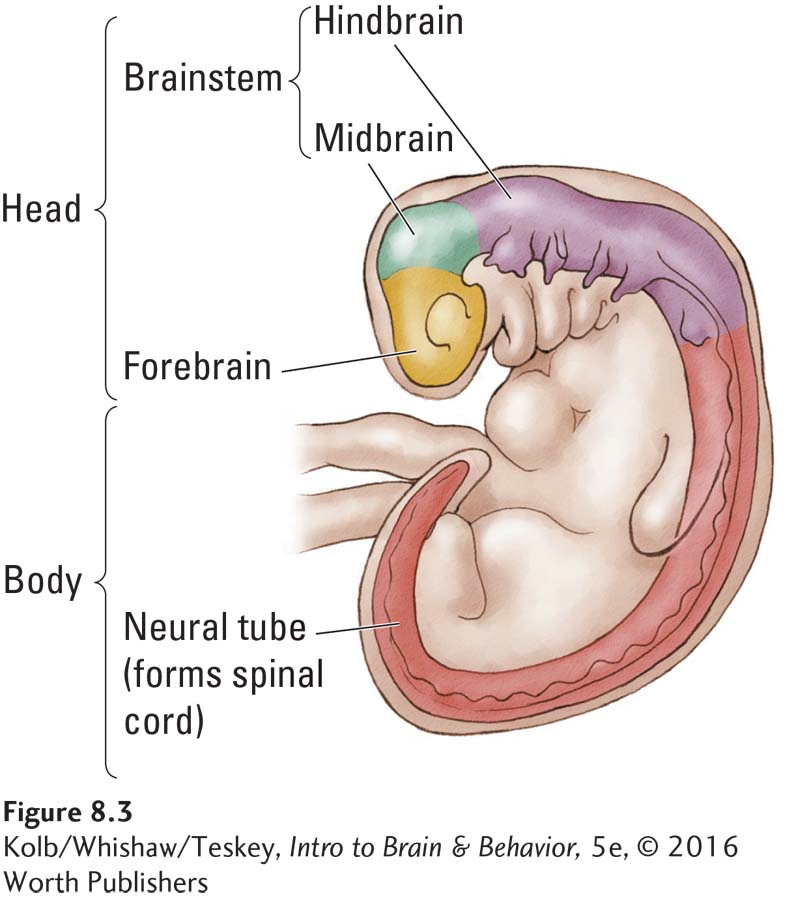
The embryonic nervous systems of vertebrates are as similar structurally as their bodies are. Figure 8-3 details the three-
Gross Development of the Human Nervous System
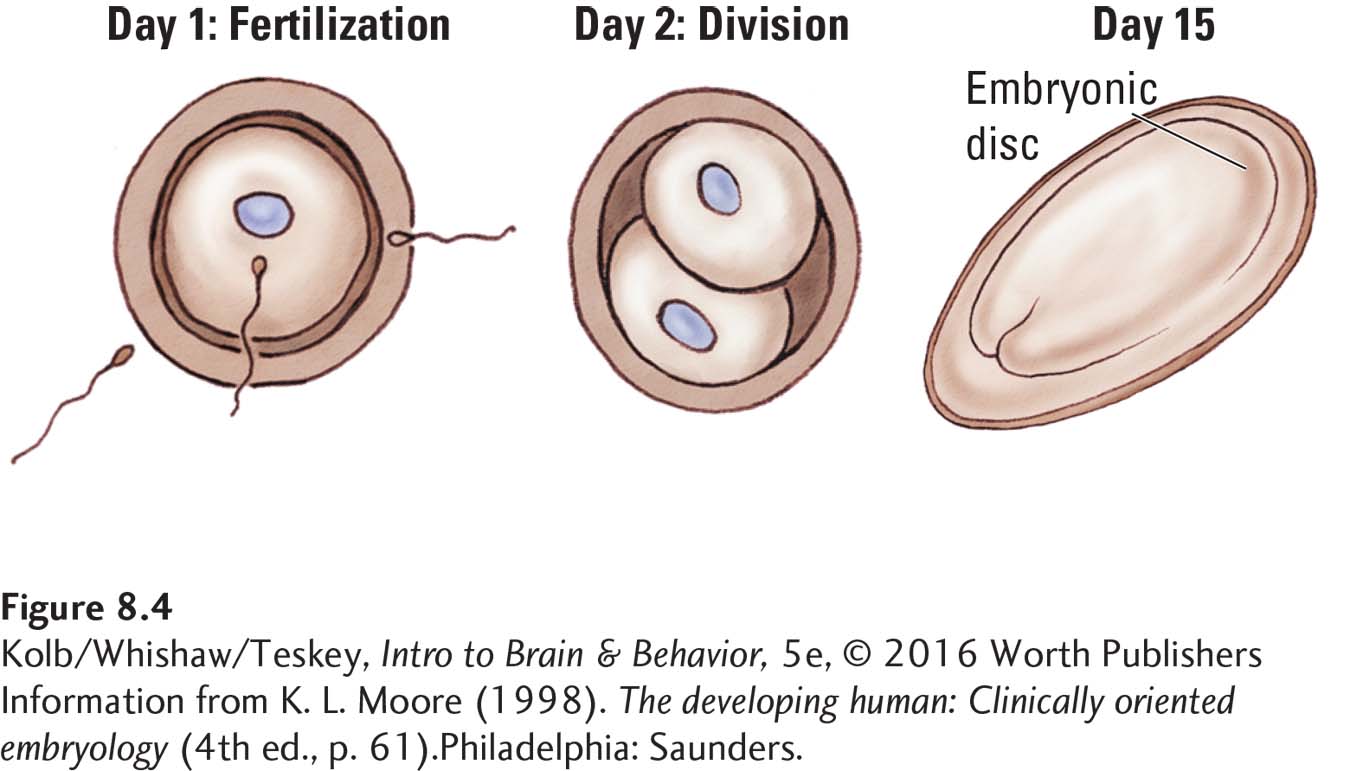
When a sperm fertilizes an egg, the resulting human zygote consists of just a single cell. But this cell soon begins to divide. By the fifteenth day after fertilization, the emerging embryo resembles a fried egg (Figure 8-4), a structure formed by several sheets of cells with a raised area in the middle called the embryonic disc—
Prenatal Stages
| Zygote | Fertilization to 2 weeks |
| Embryo | 2 to 8 weeks |
| Fetus | 9 weeks to birth |
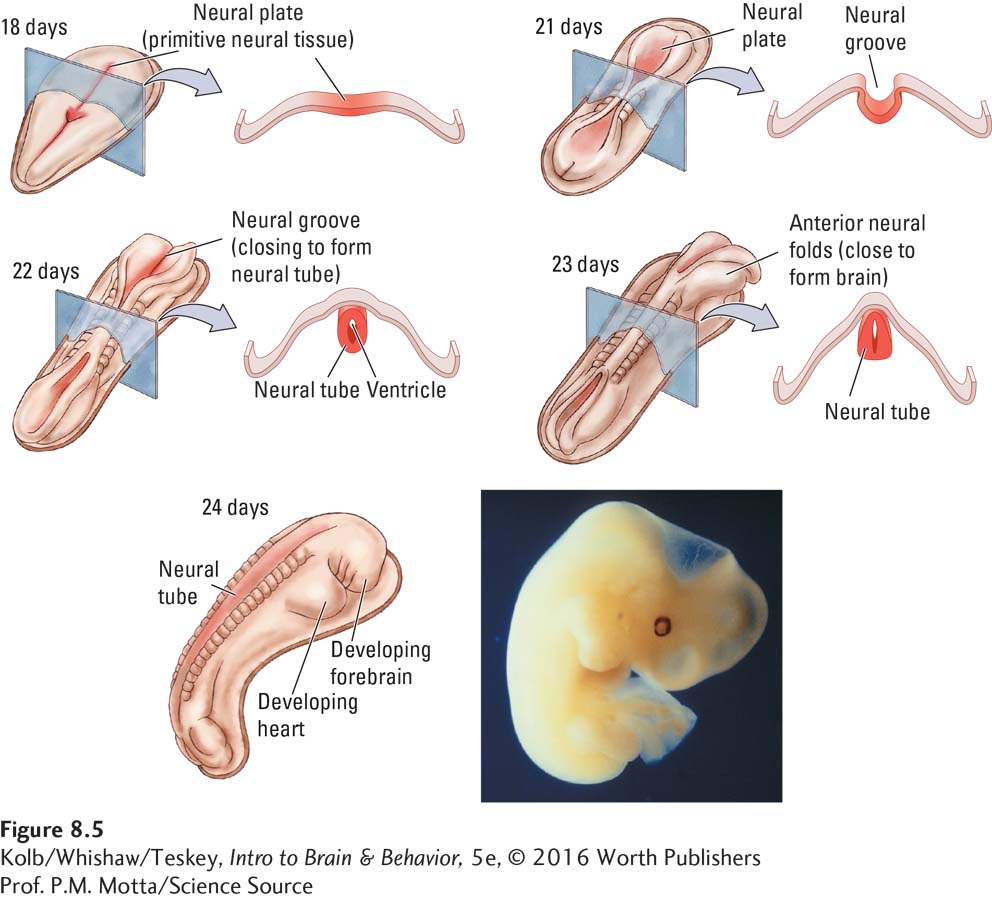
By day 21, 3 weeks after conception, primitive neural tissue, the neural plate, occupies part of the outermost layer of embryonic cells. First the neural plate folds to form the neural groove, detailed in Figure 8-5. The neural groove then curls to form the neural tube, much as you can curl a flat sheet of paper to make a cylinder.
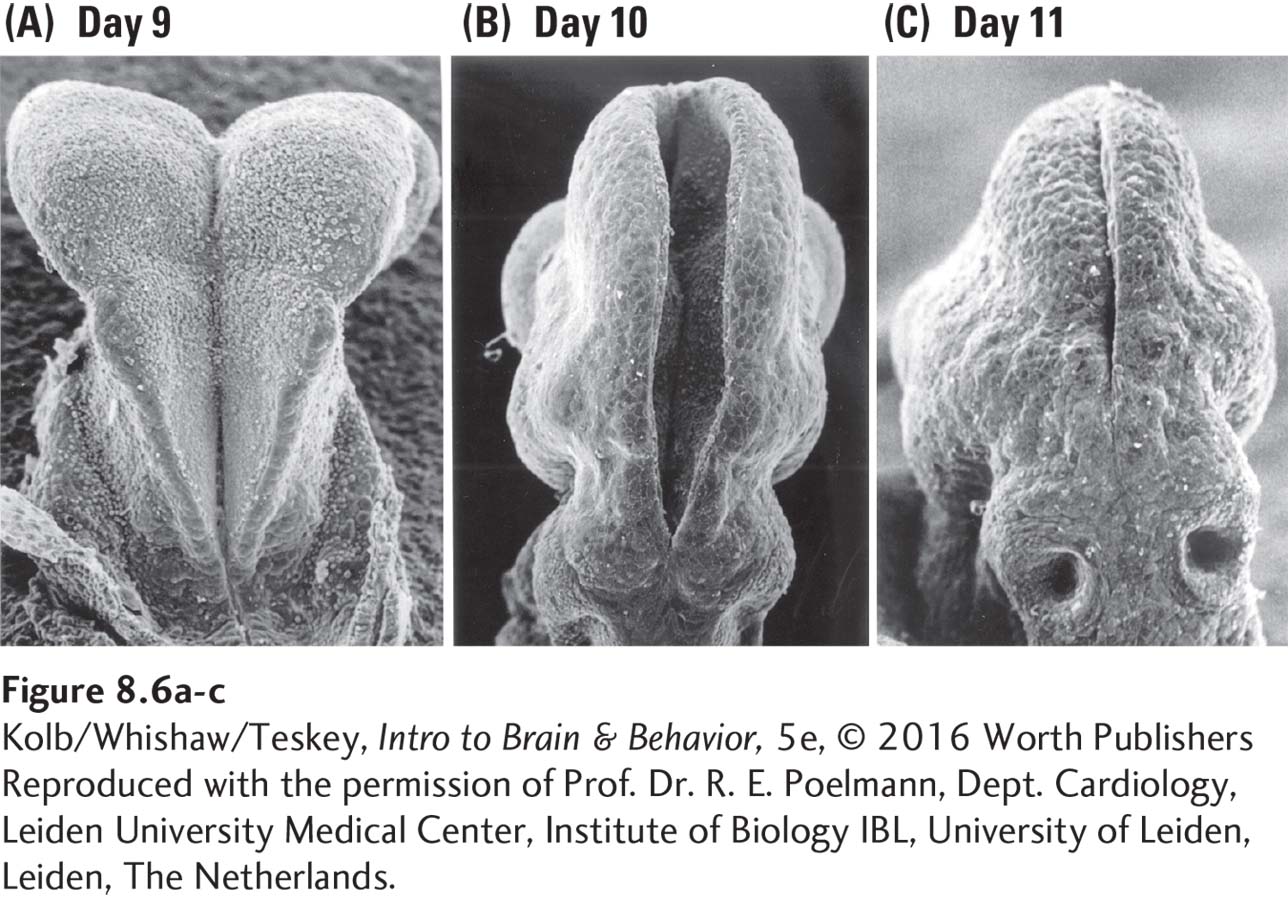
The cells that form the neural tube can be regarded as the nursery for the rest of the central nervous system. The open region in the tube’s center remains open and matures into the brain’s ventricles and the spinal canal. The micrographs in Figure 8-6 show the neural tube closing in a mouse embryo.
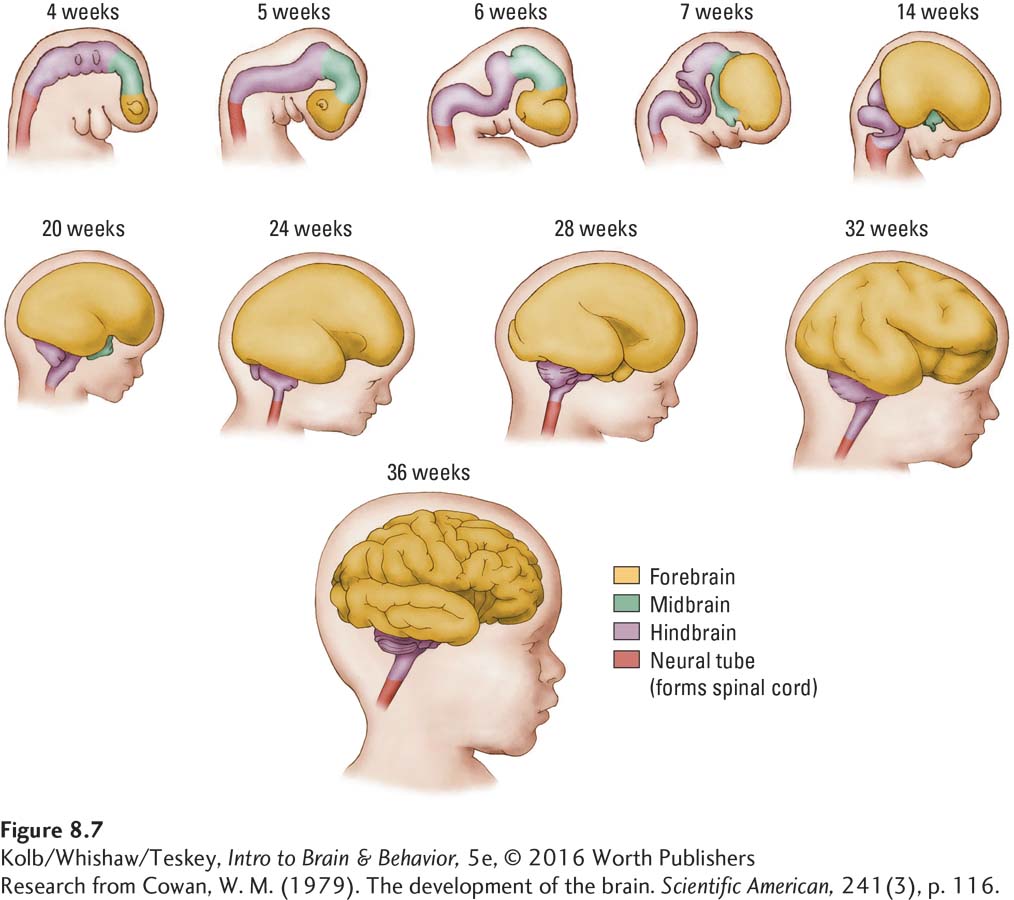
The human body and nervous system change rapidly in the next 3 weeks (Figure 8-7). By 7 weeks (49 days), the embryo begins to resemble a miniature person. The brain looks distinctly human by about 100 days after conception, but it does not begin to form gyri and sulci until about 7 months. By the end of the ninth month, the fetal brain has the gross appearance of the adult human brain, but its cellular structure is different.
Origins of Neurons and Glia
Adult stem cells that line the subventricular zone also are located in the hippocampus, spinal cord, and retina.
The neural tube is the brain’s nursery. Neural stem cells lining it have an extensive capacity for self-
If lining the ventricles were all that stem cells did throughout the decades of a human life, they would seem very odd cells to possess. But neural stem cells have a function beyond self-
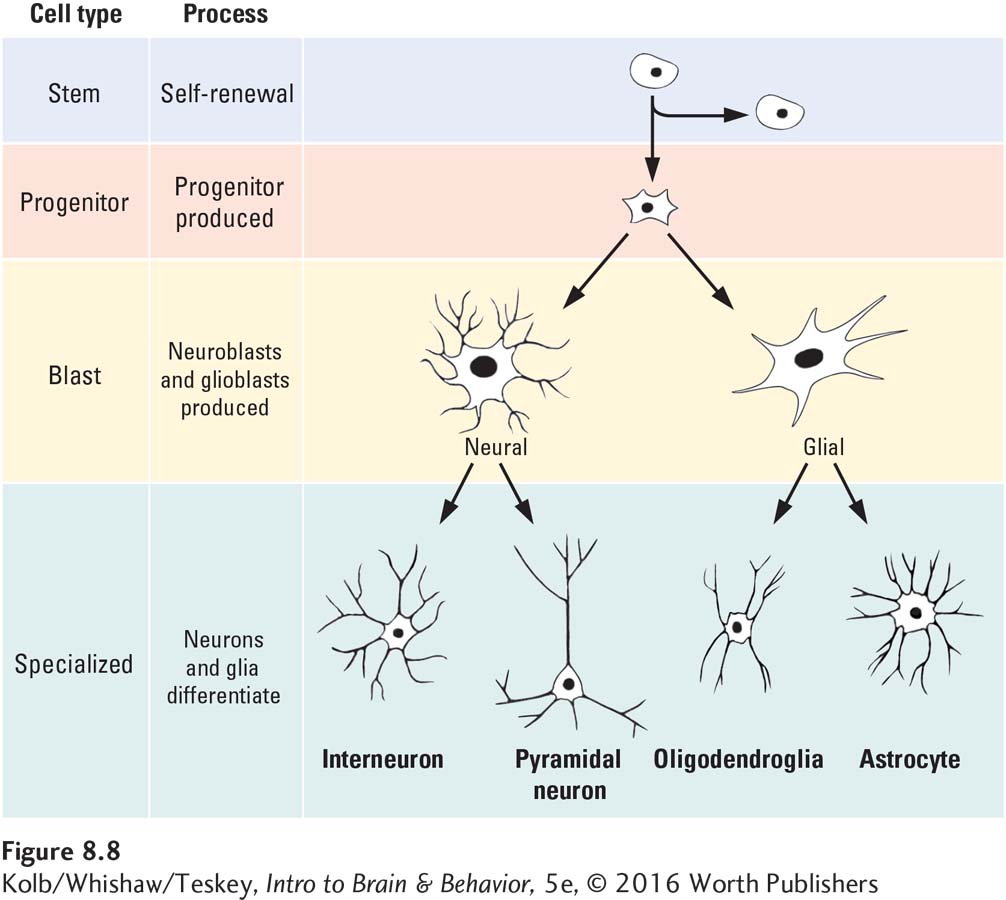
Sam Weiss and his colleagues (1996) discovered that stem cells remain capable of producing neurons and glia not just into early adulthood but even in an aging brain. This important discovery implies that neurons that die in an adult brain should be replaceable. But neuroscientists do not yet know how to instruct stem cells to replace them.
One possibility is to make use of signals that the brain typically uses to control stem cell production in adults. For example, the level of the neuropeptide prolactin increases when female mice are pregnant and stimulates the fetal brain to produce more neurons (Shingo et al., 2003). These naturally occurring hormonal signals have been shown to replace lost neurons in brain-
How does a stem cell know to become a neuron rather than a skin cell? In each cell, certain genes are expressed (turned on) by a signal, and those genes then produce a particular cell type. Gene expression means that a formerly dormant gene is activated so that the cell makes a specific protein. You can easily imagine that certain proteins produce skin cells, whereas other proteins produce neurons.
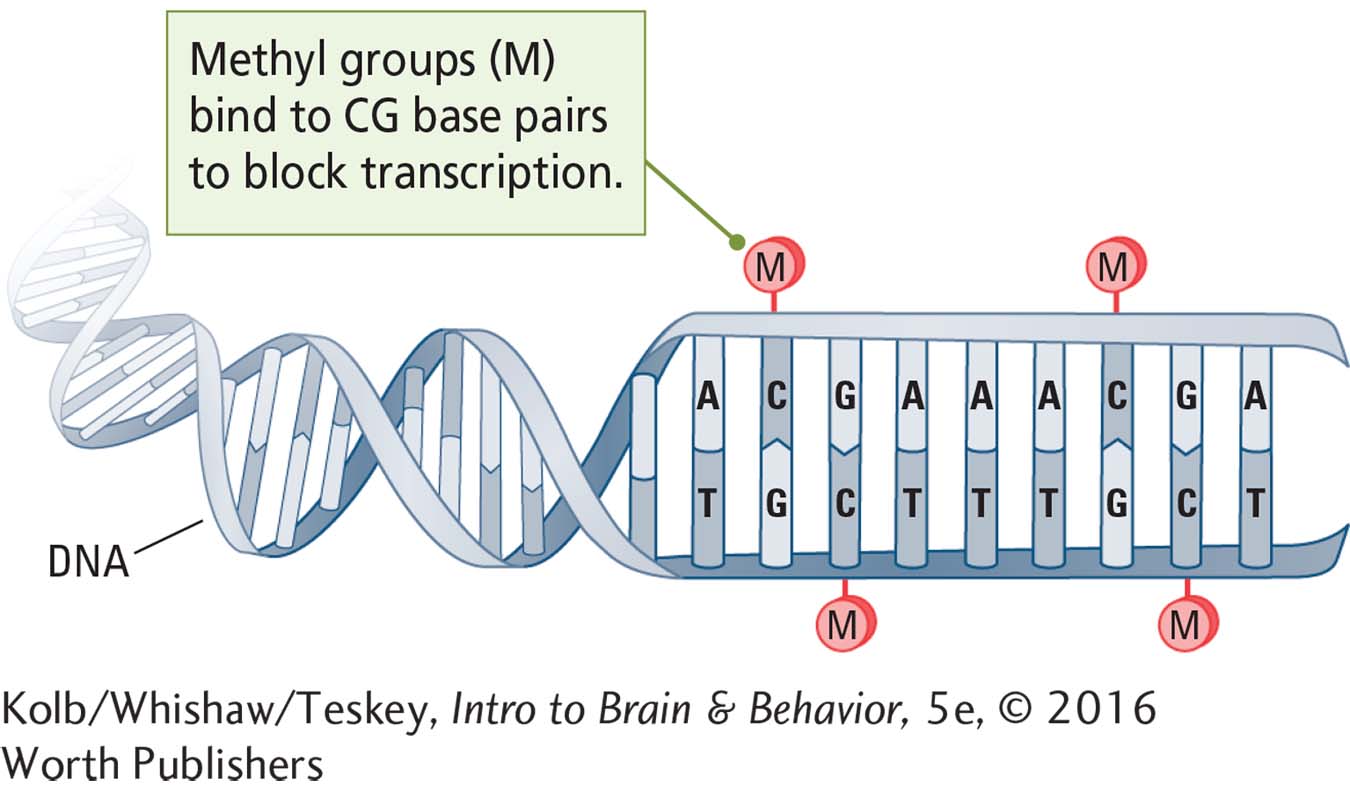
The specific signals for gene expression are largely unknown but probably are chemical, and they form the basis of epigenetics. A common epigenetic mechanism that suppresses gene expression during development is gene methylation, or DNA methylation. Here a methyl group (CH3) attaches to the nucleotide base cytosine lying next to guanine on the DNA sequence. It is relatively simple to quantify gene methylation in different phenotypes, reflecting either an increase or a decrease in overall gene expression.
Methylation alters gene expression dramatically during development. Prenatal stress can reduce gene methylation by 10 percent. This means that prenatally stressed infants express 2000 more genes (of the more than 20,000 in the human genome) than unstressed infants (Mychasiuk et al., 2011). Other epigenetic mechanisms, such as histone modification and mRNA modification, can regulate gene expression, but these mechanisms are more difficult to quantify.
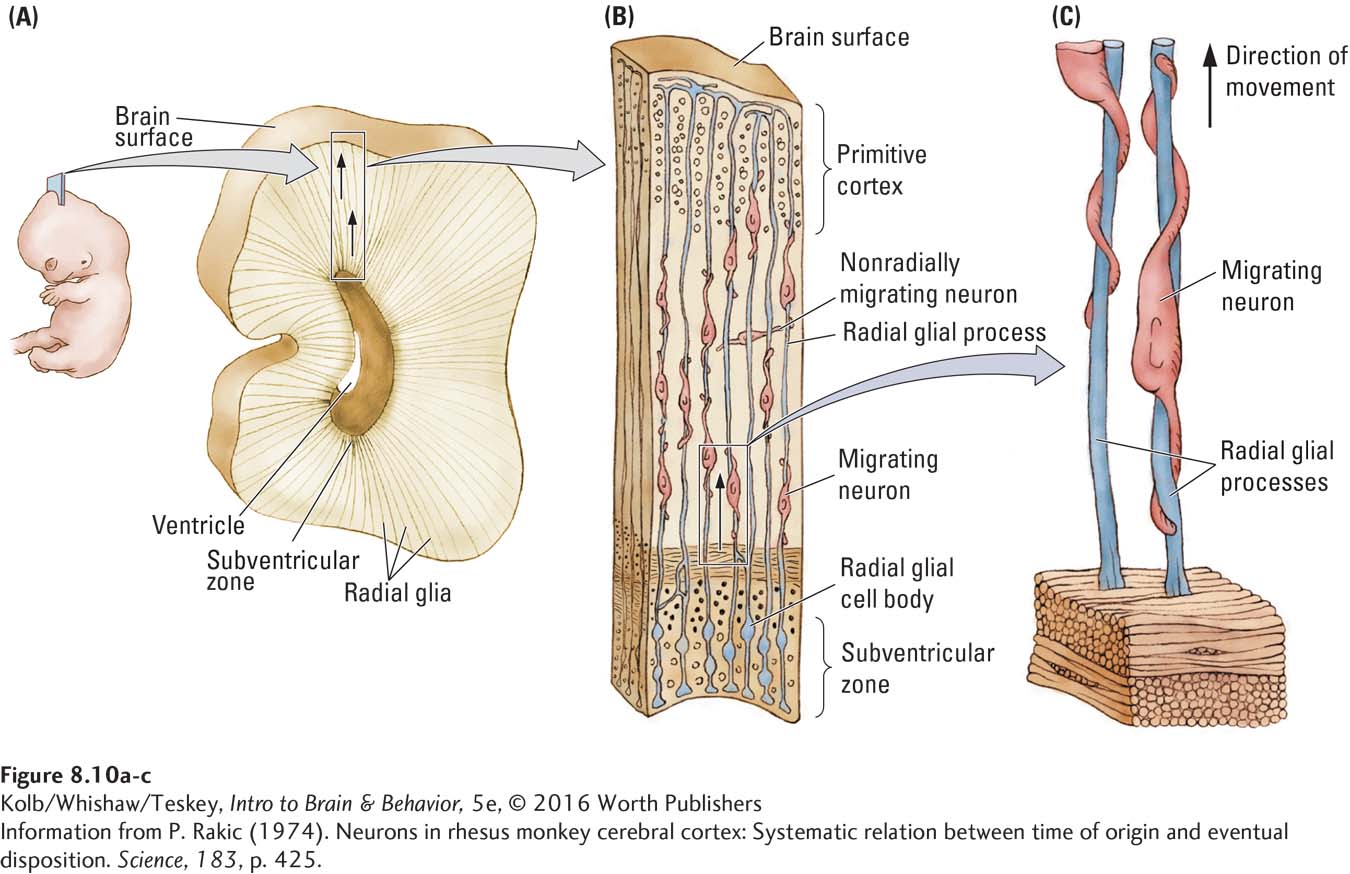
Thus, the chemical environment of a brain cell is different from that of a skin cell: different genes in these cells are activated, producing different proteins and different cell types. The chemical environments needed to trigger cellular differentiation could be produced by the activity of neighboring cells or by chemicals, such as hormones, that are transported in the bloodstream.
The differentiation of stem cells into neurons must require a series of gene-
Compounds that signal cells to develop in particular ways are neurotrophic factors (-trophic means nourishing). By removing stem cells from an animal’s brain and placing those cells in solutions that keep them alive, researchers can study how neurotrophic factors function. One compound, epidermal growth factor (EGF), when added to the stem cell culture stimulates production of progenitor cells. Another compound, basic fibroblast growth factor (bFGF, or FGF-
At this point, the destiny of a given neuroblast is undetermined. The blast can become any type of neuron if it receives the right chemical signals. The body relies on a general-
This flexibility makes brain development simpler than it would be if each different cell type, as well as the number of cells of each type, had to be specified precisely in an organism’s genes. In the same way, building a house from all-
Neuronal Growth and Development
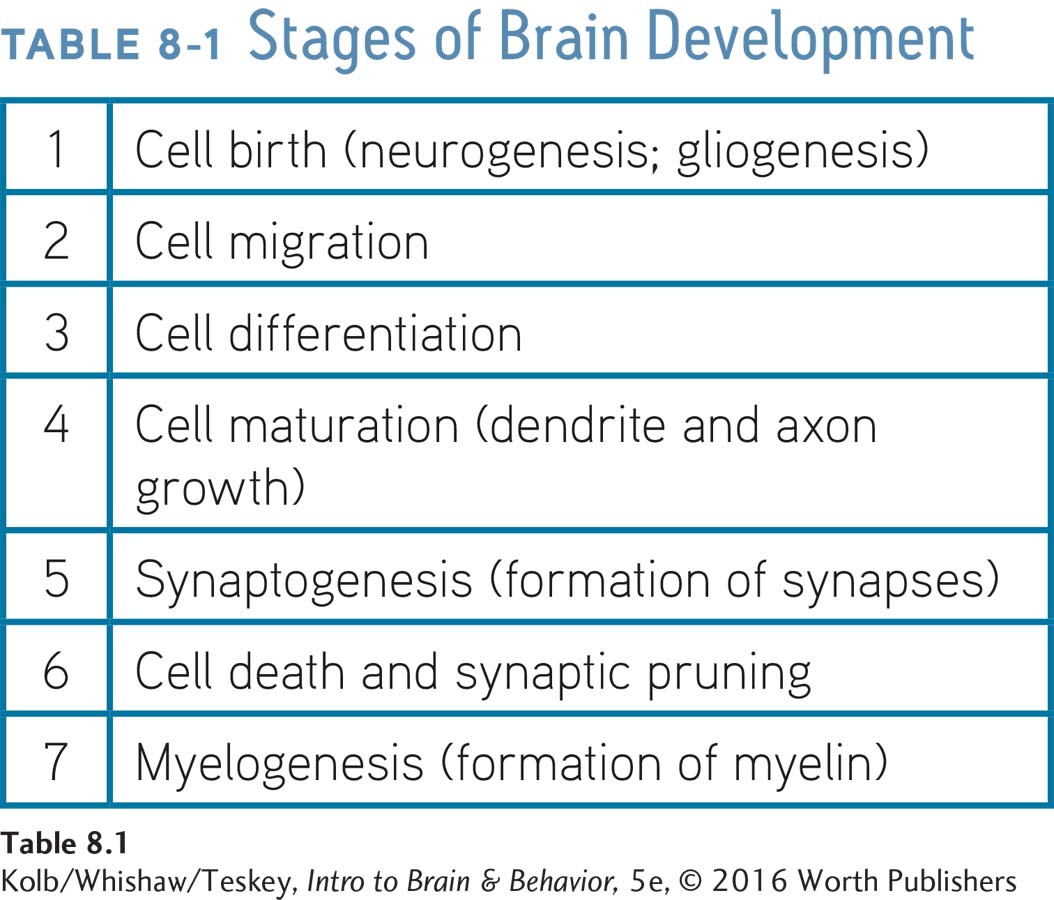
The human brain requires approximately 10 billion (1010) cells to form just the cortex that blankets a single hemisphere. This means it must produce about 250,000 neurons per minute at the peak of prenatal brain development. But as Table 8-1 shows, this rapid formation of neurons (neurogenesis) and glia (gliogenesis) is just the first step in brain growth. These new cells must travel to the correct destination (migration), they must differentiate into the right type of neuron or glial cell, and the neurons then must grow dendrites and axons and form synapses.
In Focus 8-1, investigators used cortical development as a measure of SES’s effects.
The brain also prunes unnecessary cells and connections, sculpting itself according to the particular person’s experiences and needs. We consider these stages in brain development next, focusing on cortical development, because neuroscientists know more about development of the cortex than of any other area of the human brain. The principles derived from our examination of the cortex, however, apply to neural growth and development in other brain regions as well.
Neuronal Generation, Migration, and Differentiation
Figure 8-9 shows that neurogenesis is largely complete after about 5 months of gestation. (Some growth continues until about 5 years of age.) An important exception is the hippocampus, where new neurons continue to develop throughout life.
Focus 11-2 describes outcomes resulting from cerebral palsy, caused by brain trauma acquired perinatally.
Until after full-
The hippocampus (see Figure 2-25) is critical to memory (Section 14-3) and vulnerable to stress (Section 6-5).
Cell migration begins shortly after the first neurons are generated and continues for about 6 weeks in the cerebral cortex (and throughout life in the hippocampus). Cell differentiation, in which neuroblasts become specific types of neurons, follows migration. Cell differentiation is essentially complete at birth, although neuron maturation, which includes the growth of dendrites, axons, and synapses, goes on for years and in some parts of the brain may continue throughout adulthood.
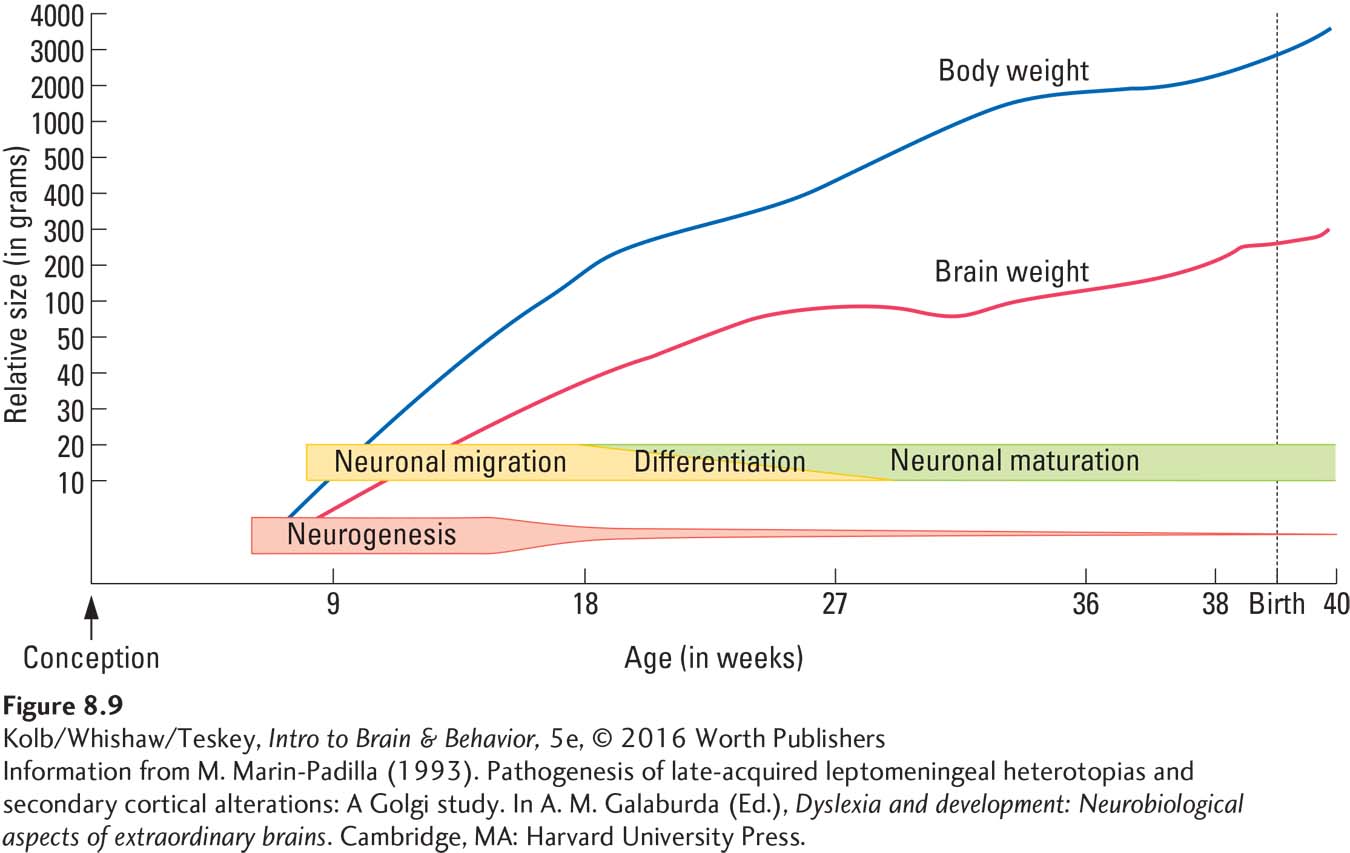
The cortex is organized into layers distinctly different from one another in their cellular makeup. How does this arrangement of differentiated areas develop? Neuroscientist Pasko Rakic and his colleagues (e.g., Geschwind & Rakic, 2013) have been finding answers to this question for more than four decades. Apparently, the subventricular zone contains a primitive cortical map that predisposes cells formed in a certain ventricular region to migrate to a certain cortical location. One subventricular region may produce cells destined to migrate to the visual cortex; another might produce cells destined to migrate to the frontal lobes, for example.
How do the migrating cells know where to find these different parts of the cortex? They follow a path made by radial glial cells. A glial fiber from each of these path-
As the brain grows, the glial fibers stretch but still go to the same place. Figure 8-10B also shows a cell moving across the radial glial fibers. Although most cortical neurons follow the radial glial fibers, a small number appear to migrate by seeking some type of chemical signal. Researchers do not yet know why these cells function differently.
Figure 2-22 contrasts the sensory and motor cortices’ six distinct layers and their functions.
Cortical layers develop from the inside out, much like adding layers to a tennis ball. The neurons of innermost layer VI migrate to their locations first, followed by those destined for layer V and so on, as successive waves of neurons pass earlier-
To facilitate house construction, each new story has a blueprint-
Local environmental signals—
Neuronal Maturation
After neurons migrate to their destination and differentiate, they begin to mature by (1) growing dendrites to provide surface area for synapses with other cells and (2) extending their axons to appropriate targets to initiate synapse formation.
Two events take place in dendrite development: dendritic arborization (branching) and the growth of dendritic spines. As illustrated in Figure 8-12, dendrites in newborn babies begin as individual processes protruding from the cell body. In the first 2 years of life, dendrites develop increasingly complex extensions that look much like leafless tree branches: they undergo arborization. The dendritic branches then begin to form spines, where most synapses on dendrites are located.
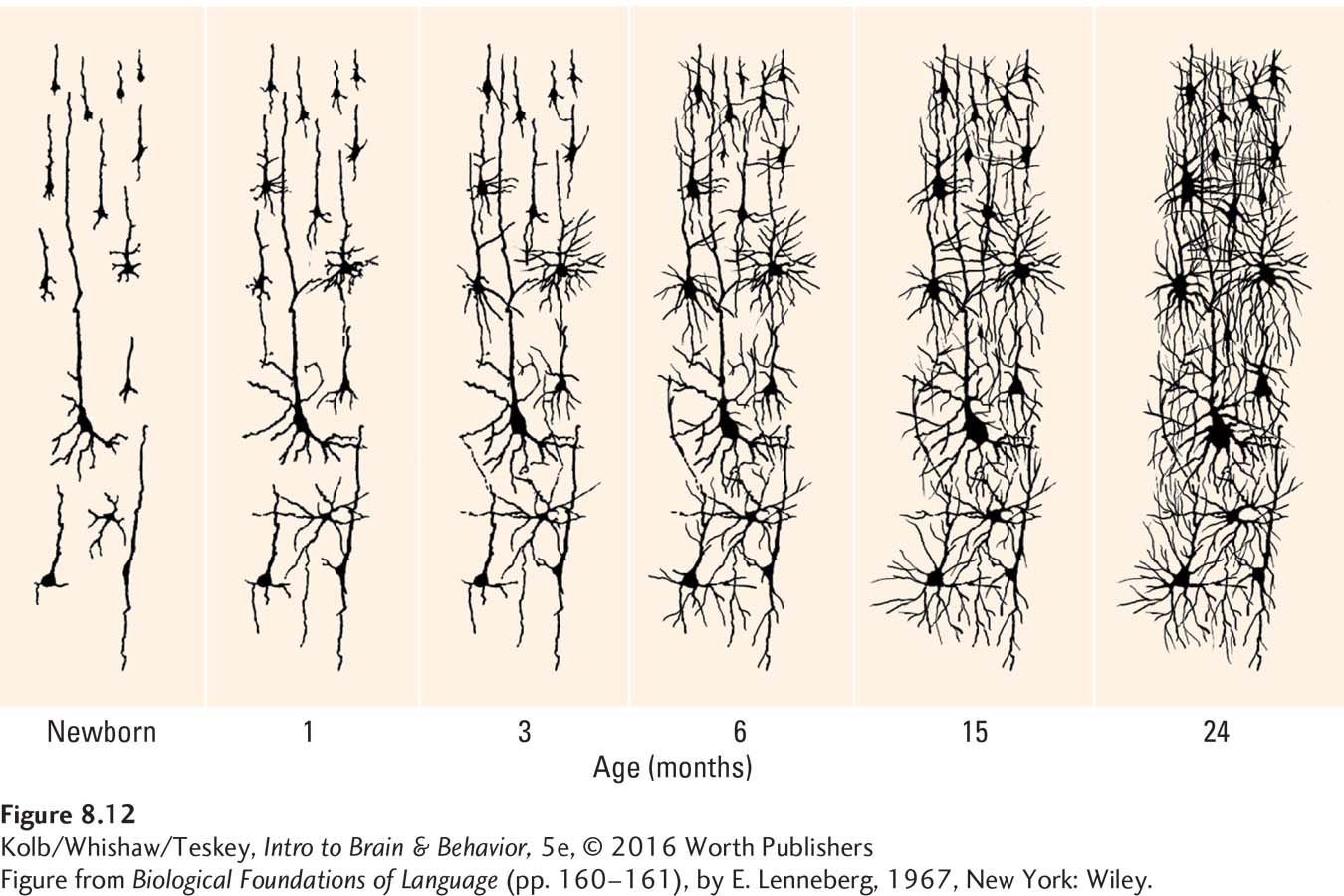
Although dendritic development begins prenatally in humans, it continues for a long time after birth, as Figure 8-12 shows. Dendritic growth proceeds at a slow rate, on the order of microns (µm, millionths of a meter) per day. Contrast this with the development of axons, which grow on the order of a millimeter per day, about a thousand times faster.
The disparate developmental rates of axons and dendrites are important, because the faster-
8-2
Autism Spectrum Disorder
In the 1940s, Leo Kanner and Hans Asperger first used the term autism (from the Greek autos, meaning self) to describe children who seem to live in their own world. Some were classified as intellectually disabled; others seemed to function intellectually.
The contemporary term, autism spectrum disorder (ASD), accommodates this behavioral range to include children with mild and severe symptoms. Severe symptoms include greatly impaired social interaction, a bizarre and narrow range of interests, marked abnormalities in language and communication, and fixed, repetitive movements.
The autism spectrum includes classic autism and related disorders. Asperger syndrome, for example, is distinguished by an obsessive interest in a single topic or object to the exclusion of nearly any other. Children with Asperger are socially awkward and also usually have delayed motor skill development. Rett syndrome, characterized by poor expressive language and clumsy hand use, almost exclusively affects girls.
The rate of ASD has been rising over the past four decades, from fewer than 1 person in 2000 in 1980 to the 2014 estimate of the Centers for Disease Control that as many as 1 in 68 children has some form of autism. The actual incidence may be as high as 1 in 50 (Blumberg et al., 2013). The cause of this increased incidence is uncertain. Suggestions include changes in diagnostic criteria, diagnosis of children at a younger age, and epigenetic influences. Although it knows neither racial nor ethnic nor social boundaries, ASD is four times as prevalent in boys as in girls.
The behavior of many children with ASD is noticeable from birth. To avoid physical contact, these babies arch their backs and pull away from caregivers or grow limp when held. But approximately one-
Perhaps the most recognized characteristics of ASD are failure to interact socially, repetitive rocking or hand flapping, impairments in language development, and resistance to any change in routine. Some children on the autism spectrum are severely impaired; others learn to function quite well. Still others display savant syndrome, a narrow range of exceptional abilities such as in music, art, or mathematics, often accompanied by severe cognitive deficits.
The brains of children diagnosed with ASD look remarkably typical. One emerging view is that these brains are characterized by unusual neuronal maturation rates. MRI studies show that at about 6 months of age, the autistic brain’s growth rate accelerates to the point that its total volume is 6 percent to 10 percent greater than that of typical children.
Excessive brain volume is especially clear in the amygdala (Nordahl et al., 2012) and in the temporal and frontal lobes, the latter showing greater gray matter volume (see the review by Chen et al., 2011). The subcortical amygdala plays an important role in generating fear, and the social withdrawal component of ASD may be related to the enlarged amygdala.
Accelerated brain growth associated with enlarged regions suggests that connections between cerebral regions are atypical, which would in turn produce atypical functioning. What leads to such brain development? More than 100 genetic differences have been described in children with ASD, so it is clear that no “autism gene” is at work.
The mechanism that translates genetic irregularities into the autistic brain is unknown but is likely to include epigenetic factors that could be prenatal, postnatal, or both. Women have an increased risk of giving birth to a child who develops ASD if they are exposed to rubella (German measles) in the first trimester of pregnancy. Researchers also suspect that industrial toxins can trigger autism, but the cause or causes remain uncertain.
No medical interventions exist for ASD. Behavioral therapies are the most successful, provided they are intense (20 to 40 hours per week) and the therapists are trained practitioners. The earlier interventions begin, the better the prognosis. Neuroscience has so far offered little insight into why behavioral therapies are effective, although in an animal model of autism, Raza et al. (2015) showed that tactile stimulation from birth until weaning reverses many morphological abnormalities in cortical neurons, suggesting a possible mechanism.
Autism may appear puzzling because no evolutionary advantage for its symptoms is apparent, but perhaps one exists. Characteristically, children with ASD are overly focused on specific tasks or information. The ability to concentrate on a complex problem for extended periods, it is suggested, is the basis for humankind’s development and for cultural advances.. But too much of such a good thing may lead to conditions such as ASD.

Axon-
Santiago Ramón y Cajal was the first scientist to describe this developmental process a century ago. He called the growing tips of axons growth cones. Figure 8-13A shows that as growth cones extend, they send out shoots, analogous to fingers reaching out to find a pen on a cluttered desk. When one shoot, a filopod (pl. filopodia), reaches an appropriate target, the others follow.
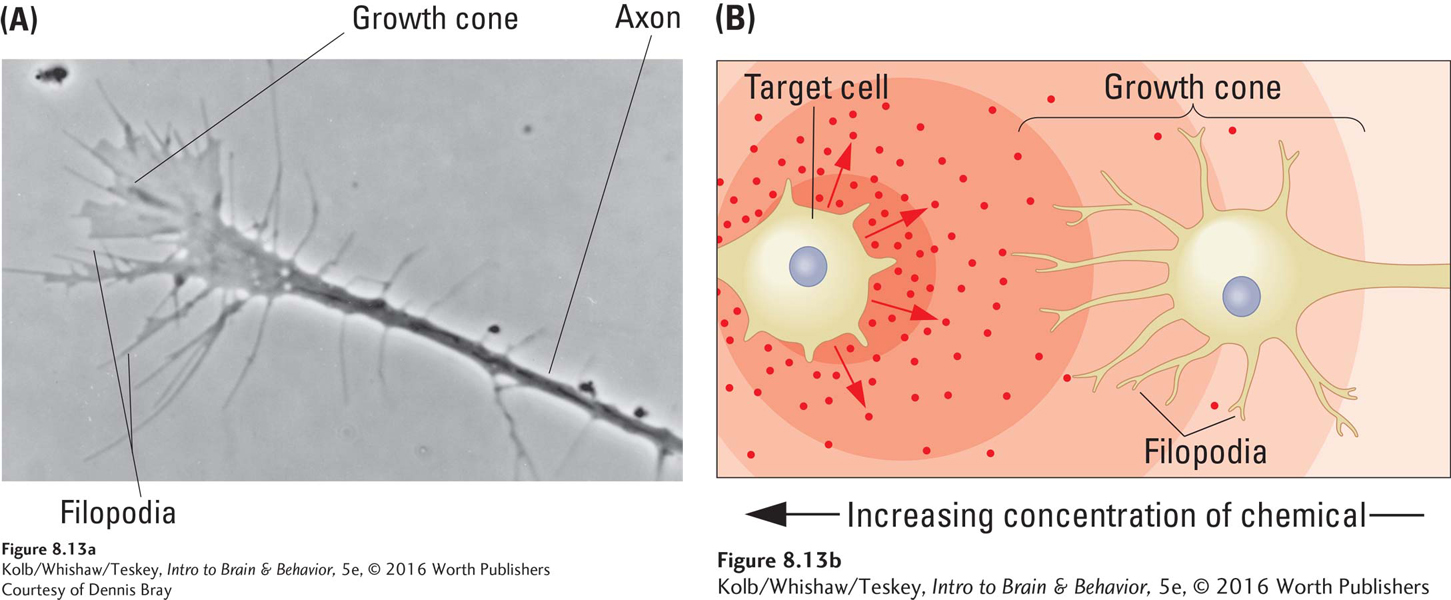
Growth cones are responsive to cues from two types of molecules (Figure 8-13B):
Cell adhesion molecules (CAMs) are cell-
manufactured molecules that either lie on the target cell’s surface or are secreted into the intercellular space. Some provide a surface to which growth cones can adhere, hence the name cellular adhesion molecule; others serve to attract or repel growth cones. Do not confuse tropic (guiding) molecules with the trophic (nourishing) molecules, discussed earlier, which support neuronal growth.
Tropic molecules, produced by the targets the axons’ growth cones are seeking (-tropic means moving toward; pronounced as trope, not tropical), essentially tell growth cones to come on over here. They likely also tell other growth cones seeking different targets to keep away.
Although Ramón y Cajal predicted them more than 100 years ago, tropic molecules have proved difficult to find. So far, only one group, netrins (from Sanskrit for to guide), has been identified in the brain. Given the enormous number of brain connections and the great complexity in wiring them, many other types of tropic molecules undoubtedly will be found.
Synaptic Development
The number of synapses in the human cerebral cortex is staggering, on the order of 1014, or 100,000 trillion. A genetic program that assigns each synapse a specific location could not possibly determine each spot for this huge number. As with all stages of brain development, only the general outlines of neuronal connections in the brain are likely to be genetically predetermined. The vast array of specific synaptic contacts is then guided into place by a variety of local environmental cues and signals.
A human fetus displays simple synaptic contacts in the fifth gestational month. By the seventh gestational month, synaptic development on the deepest cortical neurons is extensive. After birth, synapse numbers increase rapidly. In the visual cortex, synaptic density almost doubles between ages 2 months and 4 months and then continues to increase until age 1 year.
Cell Death and Synaptic Pruning
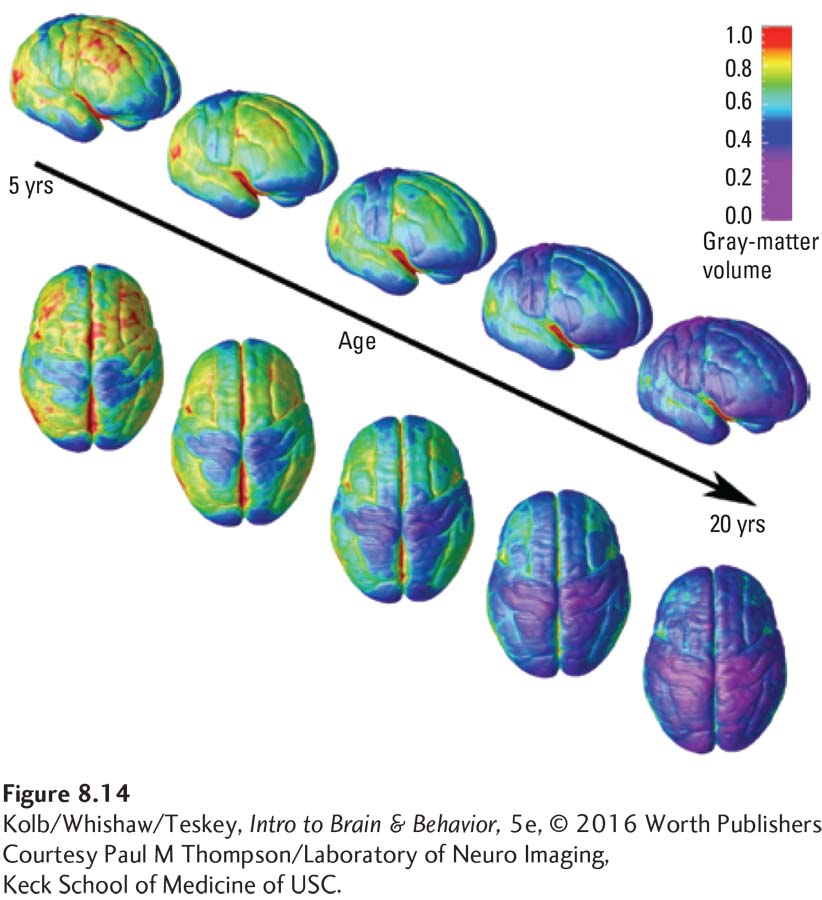
To carve statues, sculptors begin with blocks of stone and chisel away the unwanted pieces. The brain does something similar during cell death and synaptic pruning. The chisel in the brain could be a genetic signal, experience, reproductive hormones, stress, even SES. The effect of these chisels can be seen in changes in cortical thickness over time, as illustrated in Figure 8-14, an atlas of brain images. The cortex actually becomes measurably thinner in a caudal–
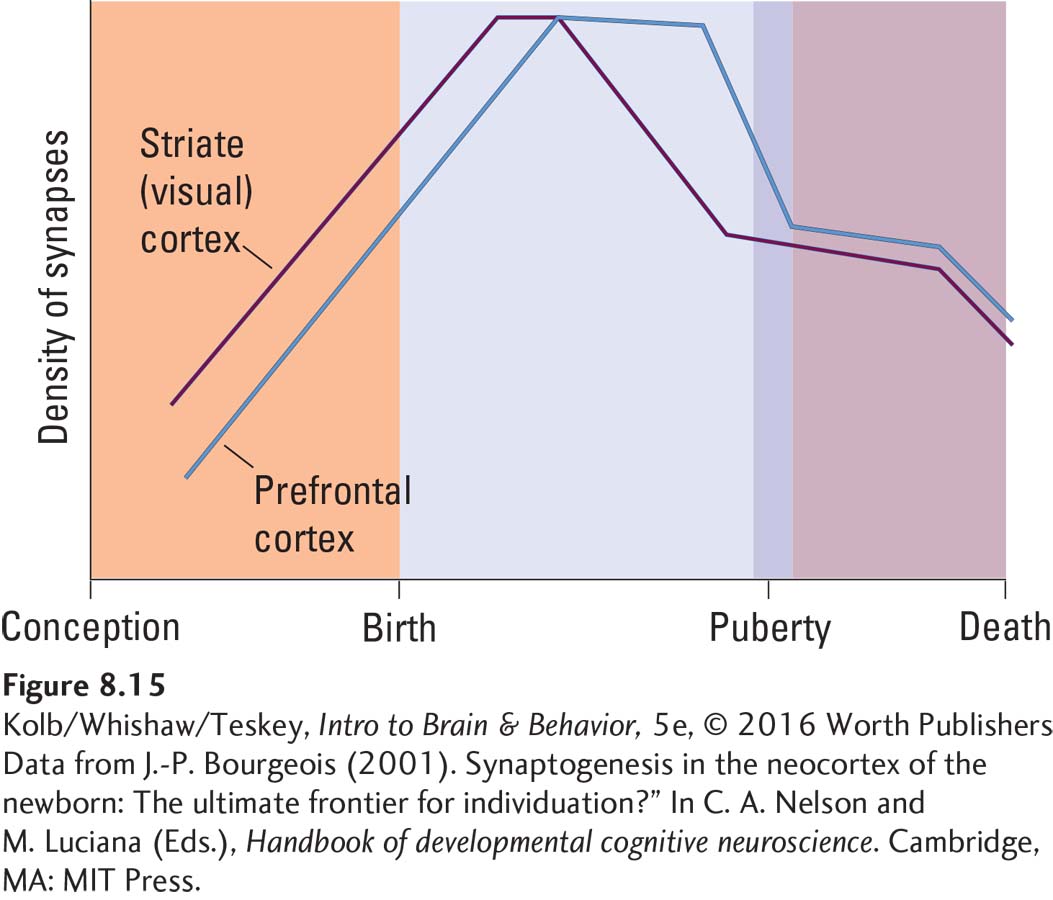
The graph in Figure 8-15 plots this rise and fall in synaptic density. Pasko Rakic (1974) estimated that at the peak of synapse loss, a person may lose as many as 100,000 per second. Synapse elimination is extensive. Peter Huttenlocher (1994) estimated it at 42 percent in the human cortex. We can only wonder what the behavioral consequence of this rapid synaptic loss might be. It is probably no coincidence that children, especially toddlers and adolescents, seem to change moods and behaviors quickly.
How does the brain eliminate excess neurons? The simplest explanation is competition, sometimes referred to as neural Darwinism. Charles Darwin believed that one key to evolution is the variation it produces in the traits possessed by a species. Those whose traits are best suited to the local environment are most likely to survive. From a Darwinian perspective, then, more animals are born than can survive to adulthood, and environmental pressures weed out the less-
What exactly causes this cellular weeding out in the brain? It turns out that when neurons form synapses, they become somewhat dependent on their targets for survival. In fact, deprived of synaptic targets, neurons eventually die. They die because target cells produce neurotrophic (nourishing) factors absorbed by the axon terminals that function to regulate neuronal survival. Nerve growth factor (NGF), for example, is made by cortical cells and absorbed by cholinergic neurons in the basal forebrain.
If many neurons compete for a limited amount of a neurotrophic factor, only some can survive. The death of neurons deprived of a neurotrophic factor is different from the cell death caused by injury or disease. When neurons are deprived of a neurotrophic factor, certain genes seem to be expressed, resulting in a message for the cell to die. This programmed process is called apoptosis.
Apoptosis accounts for the death of overabundant neurons, but it does not account for the synaptic pruning from cells that survive. In 1976, French neurobiologist Jean-
In addition to outright errors in synapse formation that give rise to synaptic pruning, subtler changes in neural circuits may trigger the same process. One such change accounts for the findings of Janet Werker and Richard Tees (1992), who studied the ability of infants to discriminate speech sounds taken from widely disparate languages, such as English, Hindi (from India), and Salish (a Native American language). Their results show that young infants can discriminate speech sounds of different languages without previous experience, but their ability to do so declines in the first year of life. An explanation for this declining ability is that synapses encoding speech sounds not typically encountered in an infant’s daily environment are not active simultaneously with other speech-
Synaptic pruning may also allow the brain to adapt more flexibly to environmental demands. Human culture is probably the most diverse and complex environment with which any animal must cope. Perhaps the flexibility in cortical organization achieved by the mechanism of selective synaptic pruning is a necessary precondition for successful development in a cultural environment.
Synaptic pruning may also be a precursor related to different perceptions that people develop about the world. Consider, for example, the obvious differences in Eastern and Western philosophies about life, religion, and culture. Given the cultural differences to which people in the East and West are exposed as their brain develops, imagine how different their individual perceptions and cognitions may be. Considered together as a species, however, we humans are far more alike than we are different.
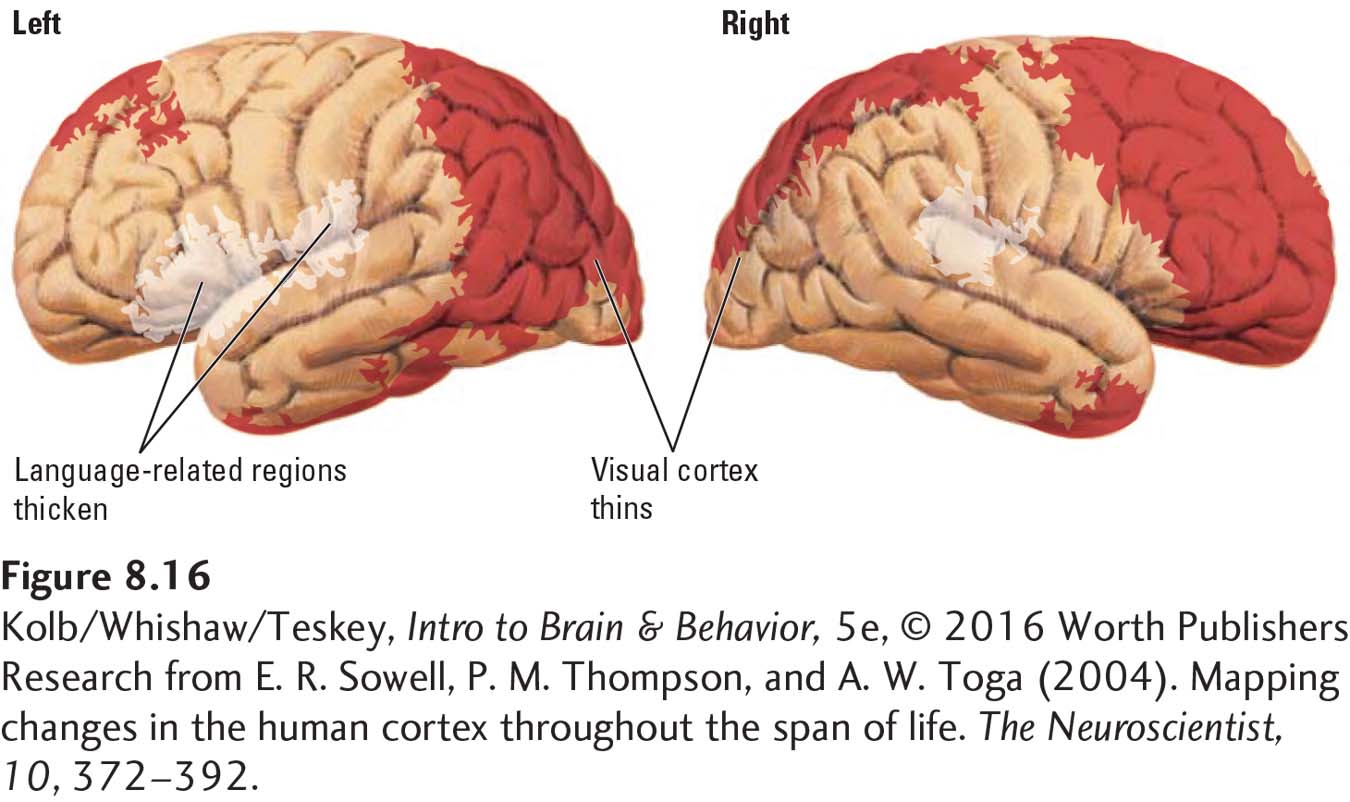
An important and unique characteristic common to all humans is language. As illustrated in Figure 8-14, the cortex generally thins from age 5 to 20. The sole exception: major language regions of the cortex actually show an increase in gray matter. Figure 8-16 contrasts the thinning of other cortical regions with the thickening of language-
Unique Aspects of Frontal Lobe Development
Three-
The imaging atlas in Figure 8-14 confirms that the frontal lobe is the last brain region to mature. Since the atlas was compiled, neuroscientists have confirmed that frontal lobe maturation extends far beyond its age 20 boundary, including in the dorsolateral prefrontal cortex. The DLPFC, which comprises Brodmann areas 9 and 46, makes reciprocal connections with posterior parietal cortex and the superior temporal sulcus: it selects behavior and movement with respect to temporal memory. Zdravko Petanjek and colleagues (2011) analyzed synaptic spine density in the DLPFC in a large sample of human brains ranging in age at death from newborn to age 91 years.
The analysis confirms that dendritic spine density, a good measure of the number of excitatory synapses, is two to three times greater in children than in adults and that spine density begins to decrease during puberty. The analysis also shows that dendritic spines continue to be eliminated well beyond age 20, stabilizing at the adult level around age 30. Two important correlates attend slow frontal lobe development:
You can view and answer the ACE questionnaire at http://www.theannainstitute.org/Finding Your ACE Score.pdf
The frontal lobe is especially sensitive to epigenetic influences (Kolb et al., 2012). In a study of more than 170,000 people, Robert Anda and colleagues (Anda et al., 2006) show that such aversive childhood experiences (ACEs) as verbal or physical abuse, a family member’s addiction, or loss of a parent are predictive of physical and mental health in middle age. People with two or more ACEs, for example, are 50 times more likely to acquire addictions or attempt suicide. Women with two or more ACEs are 5 times more likely to have been sexually assaulted by age 50. We hypothesize that early aversive experiences, such as sexual assault, promote these ACE-
related susceptibilities by compromising frontal lobe development. Abnormal frontal lobe development would make a person less likely to judge such a situation as dangerous. Page 259The trajectory of frontal lobe development correlates with adult intelligence. Philip Shaw and his colleagues (2006) used a longitudinal design, administering multiple structural MRIs to participants over time. The results show that it is not the thickness of the frontal cortex in adulthood that predicts IQ score but rather the change in trajectory of cortical thickness (Figure 8-17). Children who score highest in intelligence show the greatest plastic changes in the frontal lobe over time. These changes are likely to reflect strong epigenetic influences.
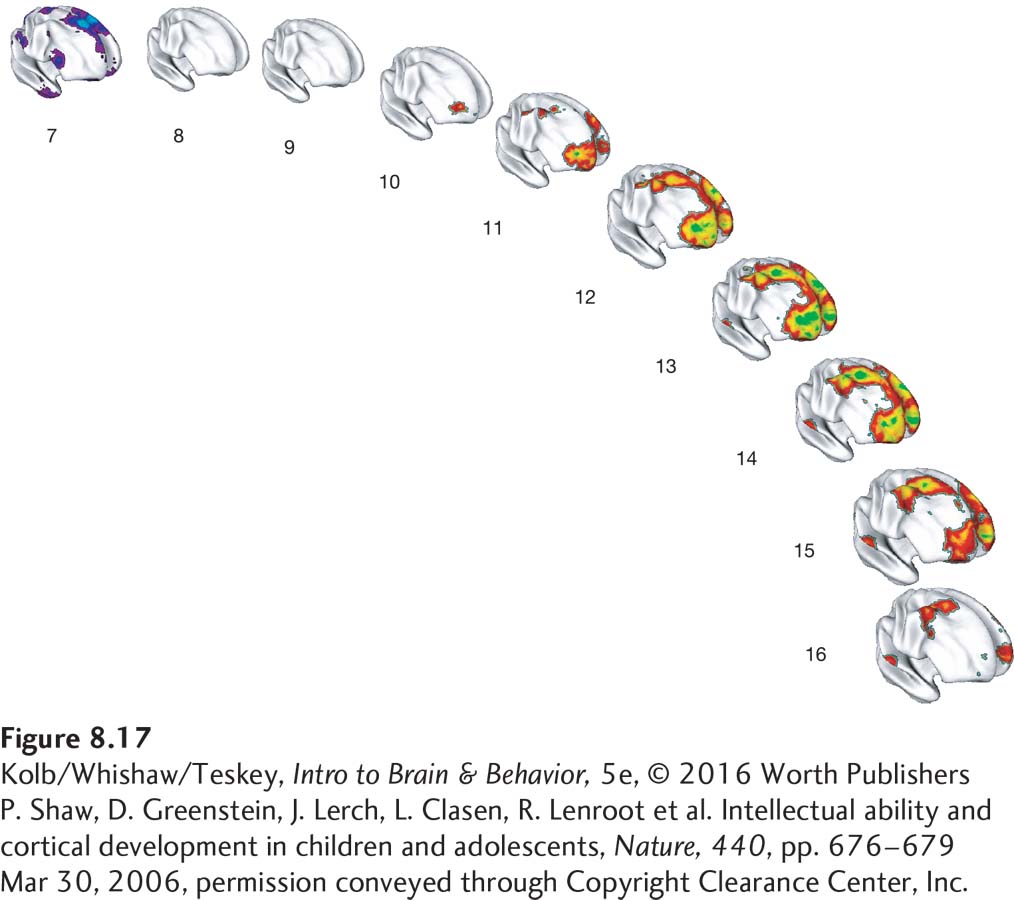 Figure 8-17: FIGURE 8-
Figure 8-17: FIGURE 8-17 Frontal Lobe Development and IQ Score The trajectory of frontal lobe development from ages 7 to 16 years correlates with dynamic changes in cortical thickness. Colors on the scans scale to the magnitude of differences between individuals with average and superior intelligence. Purple shows thinner cortex in the individuals with higher IQ scores; red, yellow, and green show progressively increasing cortical thickness in those individuals. At age 7, they have a thinner frontal cortex that rapidly thickens to peak at age 13, then wanes later in adolescence.P. Shaw, D. Greenstein, J. Lerch, L. Clasen, R. Lenroot et al. Intellectual ability and cortical development in children and adolescents, Nature, 440, pp. 676–679 Mar 30, 2006, permission conveyed through Copyright Clearance Center, Inc.
Glial Development
Astrocytes nourish and support neurons; oligodendroglia form myelin in the CNS (see Table 3-1).
Astrocytes and oligodendrocytes begin to develop after most neurogenesis is complete and continue to develop throughout life. CNS axons can function before they are myelinated by oligodendria, but healthy adult function is attained only after myelination is complete. Consequently, myelination is a useful rough index of cerebral maturation.
In the early 1920s, Paul Flechsig noticed that cortical myelination begins just after birth and continues until at least 18 years of age. He also noticed that some cortical regions were myelinated by age 3 to 4 years, whereas others showed virtually no myelination at that time. Figure 8-18 shows one of Flechsig’s cortical maps with areas shaded according to earlier or later myelination.
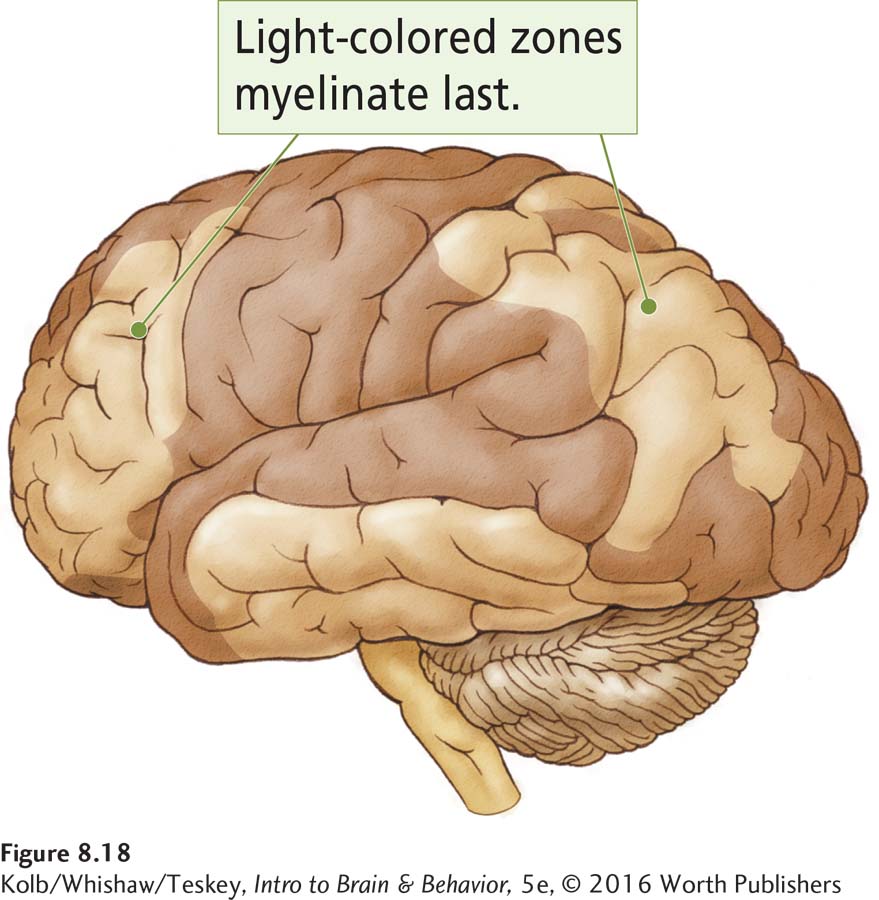
Flechsig hypothesized that the earliest-
8-2 REVIEW
Neurobiology of Development
Before you continue, check your understanding.
Question 1
The central nervous system begins as a sheet of cells, which folds inward to form the __________.
Question 2
The growth of neurons is referred to as __________, whereas formation of glial cells is known as __________.
Question 3
Growth cones are responsive to two types of cues: __________ and __________.
Question 4
The adolescent period is characterized by two ongoing processes of brain maturation: __________ and __________.
Question 5
What is the functional significance of the prolonged development of the frontal lobe?
Answers appear in the Self Test section of the book.