CONCEPT43.3 Species Are Embedded in Complex Interaction Webs
Some species are specialized in their interspecific interactions, competing with, consuming, or being consumed by few other species. Examples include leaf-cutter ants, whose entire diet consists of fungal structures; yucca moths, which pollinate and oviposit only in flowers of specific Yucca species; and many parasitic insects that attack only a single host species. However, many species are involved in interactions of many types with diverse other species. For example, most animalpollinated flowering plants are pollinated by more than one pollinator species, and few pollinators visit only one plant species. Furthermore, each species in a web of pollination interactions is connected to a much larger set of species that act as its competitors, hosts, consumers, and so forth. Prosopis shrubs (mesquite) are pollinated by many species of native bees and other insects, and their flowers and leaves are eaten, or harvested, by dozens of species of herbivorous insects, including leaf-cutter ants. Their seeds are eaten or dispersed by rodents, beetles, coyotes, foxes, and hoofed mammals, including cattle (see the opening story of Chapter 41). The roots of a Prosopis shrub interact with an entire community of soil fungi and bacteria as well as with roots of other plants. Each of the species that interacts with Prosopis in turn interacts with a constellation of other species.
Consumer–resource interactions form the core of interaction webs
These extensive webs of interspecific interactions may seem bewildering, but they make sense once we realize that they are organized around consumer–resource interactions—after all, everybody has to eat, or (to be more accurate) everybody needs energy and nutrients. Every species therefore participates in consumer–resource interactions as the consumer, the resource, or both. In addition, competition commonly occurs between consumers that use the same sources of energy and nutrients—for example, sunlight, chemical nutrients, or food—or (in the case of immobile organisms such as barnacles or plants) the space that gives them access to these resources. As we will see in Concept 43.4, even mutualisms usually involve consumers and resources. For example, leaf-cutter ants both feed and eat their fungus; algae that live inside the cells of sea anemones (see Figure 43.2) photosynthesize, producing carbohydrates that the animals consume, and in return, the anemones provide the algae with nitrogen. To be sure, energy and nutrients are not the only types of resources involved in interspecific interactions. Birds, for example, compete for nest sites that are safe for their offspring. Nevertheless, feeding, or trophic (Greek trophes, “nourishment”), interactions form the core of interaction webs.
Trophic interactions determine the flow of resources through communities. Nutrients and energy enter communities via organisms that obtain the materials that form their bodies from inorganic sources and that obtain the energy that fuels their metabolism from solar radiation or inorganic chemicals. These organisms are called primary producers because they convert energy and inorganic materials into organic compounds that can be used by the rest of the community. They are also called autotrophs (Greek, “self-feeders”) because they create their own “food” from inorganic sources (see Concept 6.6).
Species that obtain energy by breaking apart organic compounds that have been assembled by other organisms are called heterotrophs (Greek, “other-feeders”). Heterotrophs that dine on primary producers are called primary consumers, or herbivores (Latin, “plant eaters”). Those that eat herbivores are called secondary consumers, or carnivores (Latin, “flesh eaters”); those that eat the flesh of secondary consumers are called tertiary consumers, and so on. These feeding positions—primary producers and primary, secondary, and tertiary consumers—are called trophic levels. Some organisms, called omnivores, feed from multiple trophic levels. Decomposers feed on waste products or dead bodies of organisms (TABLE 43.1).
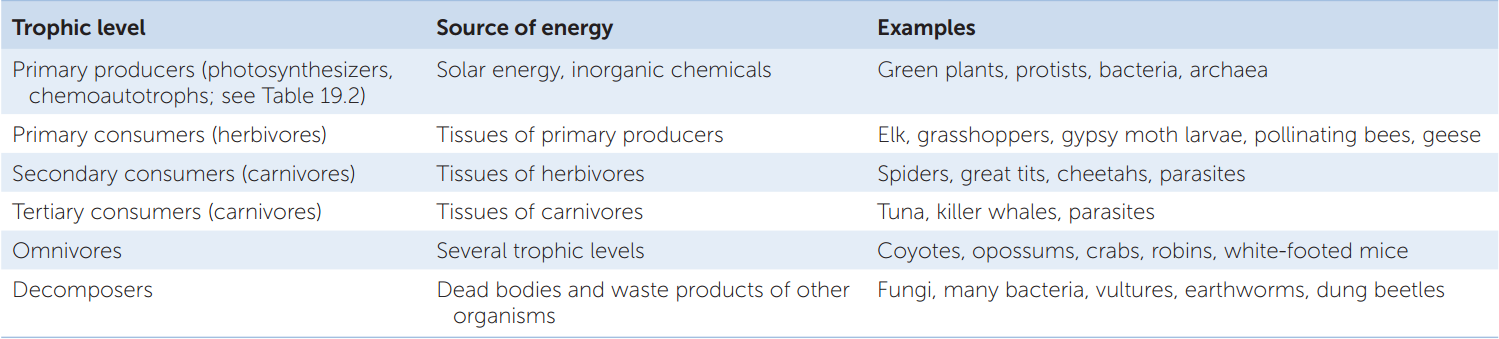
Trophic interactions can be diagrammed as a food web that shows who eats whom. Arrows from resource to consumer species show how energy and materials flow, and species are arranged vertically according to trophic level (FIGURE 43.6). Food web diagrams do not show all the interactions within a community, but they do show the major ones, and many others can be inferred from them. Competition, for example, can be inferred whenever multiple consumers use the same resource: elk (Cervus canadensis) and American bison (Bison bison) compete for grass, and plants compete for sunlight and soil nutrients.
Losses or additions of species can cascade through communities
As the naturalist and conservationist John Muir noted in his 1911 book My First Summer in the Sierra, “When we try to pick out anything by itself, we find it hitched to everything else in the Universe.” This poetic statement encapsulates an ecological fact: adding, removing, or changing the abundance of any species has effects that reverberate throughout an interaction web.
890
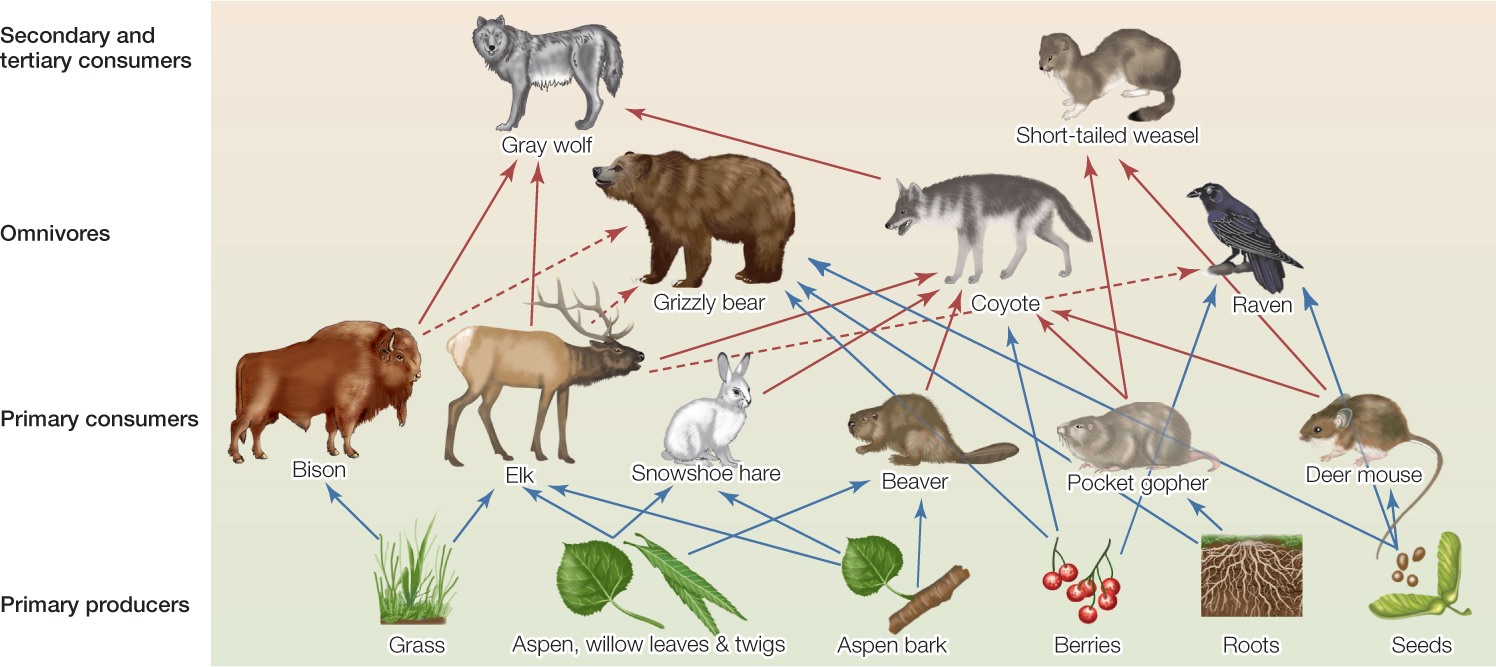
An example of what we might call the “Muir Effect” comes from Yellowstone National Park in Wyoming. Wolves (Canis lupus) in the park prey on large mammals, including elk (see Figure 43.6). Unrestricted hunting eliminated wolves from the park by 1926, and they were reintroduced in 1995. This shift in trophic interactions has had cascading effects on the entire ecosystem.
Park managers initiated annual censuses of elk in 1920. Concerned by the rapid increase in elk numbers after wolves disappeared, they deliberately kept elk numbers in check for many years. But the removal of elk ceased in 1968, and elk densities increased rapidly after that (FIGURE 43.7A). The elk browsed aspen trees (Populus tremuloides) so heavily that no young trees were added to the population after 1960 (FIGURE 43.7B). The elk also browsed streamside willows (Salix spp.), with the result that beavers (Castor canadensis), which depend on willows for food, nearly went extinct locally.
After park managers reintroduced wolves in 1995, elk populations decreased, and as a consequence, young aspen now survive, willows regrow, and species associated with these trees (beavers as well as birds, insects, and many others) have increased. This history of removal and reintroduction illuminates a cascade of effects across trophic levels—a so-called trophic cascade. What happens to predators is felt not only by their prey, but also by the prey of their prey, and by many other species connected with all of them.
891
The cascading effects of ecological interactions have implications for conservation
The Yellowstone study exemplifies the new scientific field of conservation ecology (one of many practical applications of ecology), and more specifically, the subfield of restoration ecology. Conservation ecology attempts to avoid the loss of elements or functions of an existing web of interactions, whereas restoration ecology provides scientific guidance for restoring lost elements or functions of a web. Reintroducing predators that humans once eliminated (naturally enough: a predator that eats our own prey or domestic animals is our competitor!) is a bold attempt to restore ancestral ecosystems. It also illustrates a bigger point: if we wish to restore or conserve ecological systems, we must consider the entire web of ecological interactions. And if we strive to conserve a particular species that is in danger of extinction, we must consider the web from the standpoint of that species, looking outward at all of its ecological interactions.
Nothing illustrates these points better than the problem of species introductions. As we saw in Concept 41.5, humans are introducing many species to new places, both intentionally and unintentionally. These introductions not only blur biogeographic boundaries but also have the potential to alter interactions among native species. Species that are introduced into a region where their natural enemies are absent may reproduce rapidly and spread widely. Such invasive species are likely to have negative effects on native species that lack adaptations to compete with or defend themselves against the newcomers. Alternatively, introduced species may benefit some native species if they provide a resource that was not available previously. In all cases, they alter interaction webs, as we will see in Chapter 44.
Introduced invasive species can harm native species in several ways. Introduced yellow star-thistle (Centaurea solstitialis), for example, grows rapidly in grasslands and physically crowds out native species, covering entire areas with its spiny flowering heads (see p. 874). Other flowering plants alter relationships between native plants and their mutualists. Purple loosestrife (Lythrum salicaria), introduced into North America in the early 1800s, not only crowds out natives but also—adding insult to injury—competes with them for the attention of insect pollinators. The native species Lythrum alatum, for example, receives fewer visits from bumble bees, and produces fewer seeds as a result, when purple loosestrife is present.
Some invaders alter ecological interactions by causing extinctions of native species. Concept 22.3 described the case of an introduced sac fungus, a pathogen that drove the oncedominant American chestnut (Castanea dentata) to extinction. The chestnut has been replaced by oaks throughout the forests of the northeastern United States. Because acorn production varies greatly from year to year, whereas chestnuts produced consistent nut crops every year, the loss of chestnuts has precipitated dramatic year-to-year fluctuations in rodents, ticks, and Lyme disease (see Figure 42.2).
892
Organisms that are deliberately introduced to control pests can alter interactions among native species if they do not confine their attention to the pest species. Rhinocyllus conicus, for example, is a Eurasian weevil whose larvae eat developing seeds. It was introduced into North America in 1968 to control the invasive musk thistle (Carduus nutans). But when the abundance of musk thistle declined, the weevil moved on to the native Platte thistle (Cirsium canescens) and then to wavyleaf thistle (C. undulatum). The introduced weevil has become not only a consumer of native thistles but also a competitor of the native insects that eat thistles.
CHECKpointCONCEPT43.3
- Why do some introduced species become invasive?
- At what trophic level would you place humans?
- What would a terrestrial landscape look like if its communities contained no decomposers?
The effects of interspecific and intraspecific interactions on individual members of a population depend on those individuals’ characteristics. For instance, if individuals vary in heritable attributes that determine how much a competitor affects their access to resources, or that determine their risk of being eaten by a predator, then the interaction will result in evolution by natural selection.