CONCEPT6.6 Photosynthetic Organisms Use Chemical Energy to Convert CO₂ to Carbohydrates
The energy in ATP and NADPH is used in the carbon-fixation reactions to “fix” CO2 into a reduced form and convert it to carbohydrates. Most CO2 fixation occurs only in the light, when ATP and NADPH are being generated. The metabolic pathway occurs in the stroma, or central region, of the chloroplast (see Figure 6.15) and is called the Calvin cycle after one of its discoverers, Melvin Calvin.
Like all biochemical pathways, each reaction in the Calvin cycle is catalyzed by a specific enzyme. The cycle is composed of three distinct processes (FIGURE 6.22):
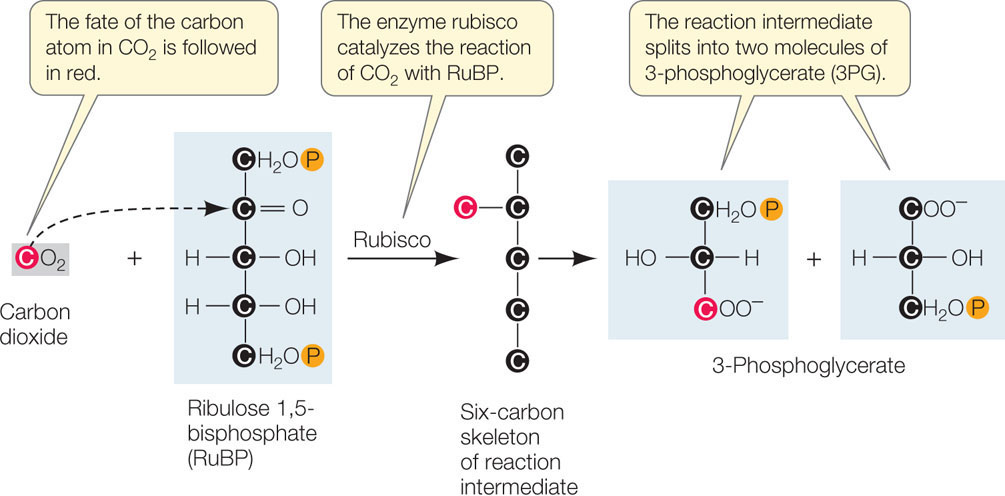
- Fixation of CO2. The initial reaction of the Calvin cycle adds the one-carbon CO2 to an acceptor molecule, the five-carbon ribulose 1,5-bisphosphate (RuBP). The immediate product is a six-carbon molecule, which quickly breaks down into two three-carbon molecules called 3-phospho-glycerate (3PG; FIGURE 6.23). The enzyme that catalyzes this reaction, ribulose bisphosphate carboxylase/oxygenase (rubisco), is rather sluggish as enzymes go. It typically catalyzes two to three fixation reactions per second. Because of this, plants need a lot of rubisco to perform enough photosynthesis to satisfy the needs of growth and metabolism. Rubisco constitutes about half of all the protein in a leaf, and it is probably the most abundant protein in the world!
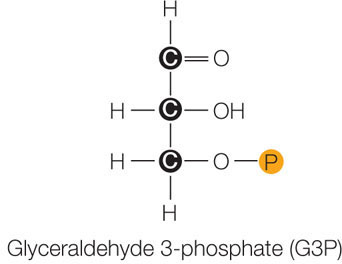
- Reduction of 3PG to form glyceraldehyde 3-phosphate. This series of reactions involves a phosphorylation (using the high-energy phosphate from an ATP made in the light reactions) and a reduction (using an NADPH made in the light reactions). The product is glyceraldehyde 3-phosphate (G3P), which is a three-carbon sugar phosphate, also called triose phosphate:
- Regeneration of the CO2 acceptor, RuBP. Most of the G3P ends up as ribulose monophosphate (RuMP), and ATP is used to convert this compound into RuBP. So for every “turn” of the Calvin cycle, one CO2 is fixed and the CO2 acceptor is regenerated.
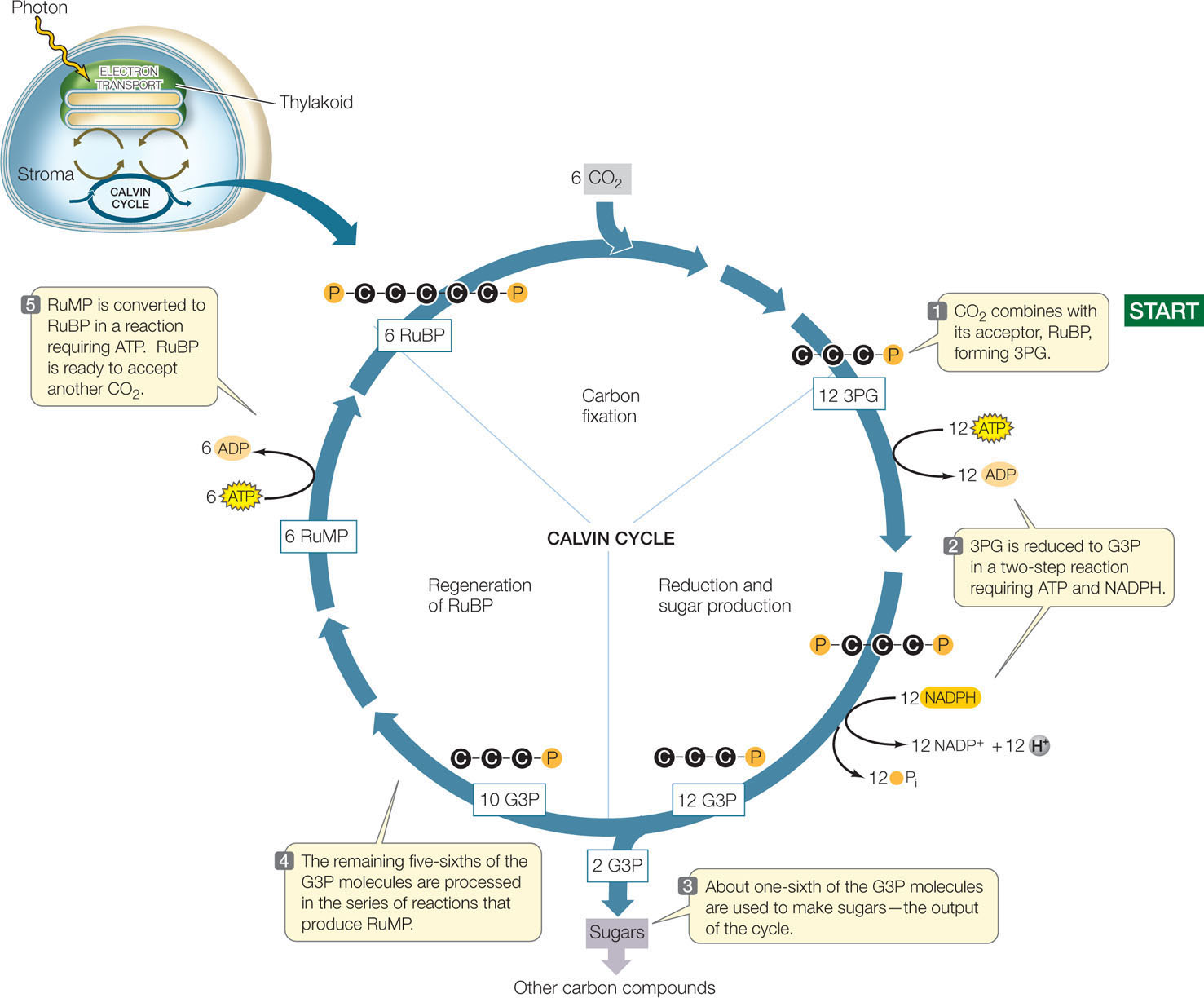
What happens to the extra G3P made by the Calvin cycle (see Figure 6.22)? It has two fates, depending on the time of day and the needs of different parts of the plant:
- Some of the extra G3P is exported out of the chloroplast to the cytosol, where it is converted to hexoses (glucose and fructose). This is the familiar C6H12O6 from the general equation for photosynthesis. These molecules may be catabolized for energy in mitochondria as part of cellular respiration; used as carbon skeletons for the synthesis of amino acids and other molecules (see Figure 6.13); or converted to sucrose, which is transported out of the leaf to other organs in the plant.
- Late in the day when glucose has accumulated inside the chloroplast, the glucose units are linked to form the polysaccharide starch. This storage carbohydrate can then be drawn on during the night so that the photosynthetic tissues can continue to export sucrose to the rest of the plant, even when photosynthesis is not taking place. In addition, starch is abundant in nonphotosynthetic organs such as roots, underground stems, and seeds, where it provides a ready supply of glucose to fuel cellular activities, including plant growth.
The products of the Calvin cycle are of crucial importance to Earth’s entire biosphere. The C—H covalent bonds generated by this cycle store almost all of the energy for life on Earth. Photosynthetic organisms, which are also called autotrophs (“self-feeders”), release most of this energy in cellular respiration, and use it to support their own growth, development, and reproduction. But plants are also the source of energy for other organisms. Much plant matter ends up being consumed by heterotrophs (“other-feeders”), including humans and other animals, which cannot photosynthesize. Heterotrophs depend on autotrophs for chemical energy, which they harvest via cellular respiration. In addition, many heterotrophs rely on plants to produce other molecules (such as vitamins) that the heterotrophs cannot synthesize for themselves.
LINK
Concept 44.3 describes how the energy captured by autotrophs flows through ecological communities
125
126
CHECKpointCONCEPT6.6
- What are the three processes of the Calvin cycle?
- If green plant cells are incubated in the presence of CO2 molecules containing radioactive carbon atoms, the fate of the carbon atoms can be followed. In an experiment, radioactive CO2 was given for 1 minute to plant cells, and then the cells were examined after 1, 5, 10, 20, and 30 minutes. The following molecules were labeled with radioactive carbon at some point(s): glucose, glyceraldehyde 3-phosphate, glycine (an amino acid), 3-phospho-glycerate, ribulose 1,5-bisphosphate, and sucrose. List these molecules in the order in which they first become labeled.
Why does fresh air inhibit the formation of alcohol by yeast cells?
ANSWER Armed with our knowledge of metabolism, we can now explain Pasteur’s observations of beet sugar and alcohol:
- Nothing happened to beet mash unless microscopic yeast cells were present. Beet sugar is a product of photosynthetic CO2 fixation (Concept 6.6). Catabolism of carbohydrates is a cellular activity, characteristic of virtually all living things (Concepts 6.2 and 6.3).
- In the presence of fresh air, yeast cells grew vigorously on the mash, and bubbles of CO2 were formed. Carbohydrate catabolism under aerobic conditions yields a large amount of ATP (Concepts 6.1 and 6.2), which can be used to fuel anabolic pathways for growth (Concept 6.4). Under aerobic conditions, glucose is fully oxidized to CO2 (Concept 6.2).
- Without fresh air, the yeast grew slowly, less CO2 was produced, and alcohol was formed. Alcoholic fermentation occurs in anaerobic conditions, and yields far less ATP (for growth) than aerobic metabolism; a small amount of CO2 is produced (Concept 6.3).
As we noted in the opening story, the use by humans of yeasts for fermentation has a long history (FIGURE 6.24).

Beer comes from the fermentation of barley seeds. These are soaked to begin the process of germination, which includes the induction of an enzyme that hydrolyzes the starch stored in the seed. The resulting disaccharide, maltose, is what the yeast cells use for energy—they first break the maltose down to its glucose monomers. At a cool temperature and under anaerobic conditions, yeast produces alcohol. An herb called hops is added to impart a distinctive bitter flavor.
Wine grapes are crushed, and the resulting juice contains sugars that are used by yeast for fermentation. There are yeasts growing on the skins of grapes naturally, so the original winemakers did not add yeast. More recently, special yeast strains have been used to control the fermentation process. The longer this process is allowed to proceed, the less sugar remains (resulting in a less sweet taste) and the higher the alcohol content. After fermentation the wine is stored in wooden casks, typically at cool temperatures. During this time hundreds of molecular transformations occur, giving different wines distinctive properties.
Bread is made from ground-up plant seeds (flour), which contain abundant starch. Moisture activates enzymes inside the seed that catabolize the starch to monosaccharides. These monosaccharides are used by bread yeast in fermentation reactions that result in CO2 production, and the resulting gas bubbles cause the complex flour mixture to rise.
127