CONCEPT43.4 Interactions within and among Species Can Result in Evolution
Both intraspecific and interspecific interactions can affect the success of an average individual in surviving and reproducing. But averages tell us only part of the story, because no individual is exactly average. Interactions will therefore affect individuals to different extents, causing them to vary in their contribution to population growth rate—that is, in their fitness. Heritable traits that cause greater benefit from positive interactions, or less harm from negative interactions, will become more common in the population. The dynamics of this evolutionary change (see Chapter 15) will depend on the mix of positive and negative effects that characterize an interaction. Let’s consider some examples, starting with intraspecific competition.
Intraspecific competition can increase the carrying capacity
Equations for density-dependent population growth, such as the word equation in Concept 42.4, assume that all the individuals in a population are equally affected by its density. However, individuals often vary in phenotypic traits that affect how well they can use available resources. Consider the seed-eating finches of the Galápagos archipelago off the west coast of Ecuador (see Figure 17.7). The average beak size for any given finch species varies from island to island, and the beak size on any given island is close to the size that is best for processing one of the types of seeds available on the island (FIGURE 43.8). Such patterns suggest that beak size has evolved in response to the available seeds. To visualize this process, consider the following scenario.
LINK
Three patterns of natural selection—directional, stabilizing, and disruptive—are described in Concept 15.4
Investigation
HYPOTHESIS
Differences in the morphology of finches among islands reflect interisland differences in the availability of seeds of different sizes.
- Determine which species of seeds can be eaten by Geospiza species with different beak sizes, and determine the species’ food requirements.
- On each island, measure the abundances of seeds of different plant species and calculate the availability of food for finches of different beak sizes.
- From these measurements, calculate for each island how many finches of a given beak size could be supported if no other finch species were present (this is an estimate of carrying capacity). Do this for hypothetical finches with beak sizes that span the possible range for seed-eating Geospiza species.
- Display the calculated results as a graph for each island, with beak size on the x axis and estimated carrying capacity on the y axis. A peak in the graph predicts a high carrying capacity for a finch species with that beak size. A low point means the opposite—a species with that beak size is predicted to achieve only a low equilibrium density.
- On each graph, place a dot showing the mean beak size of each Geospiza species actually present on the island.
- Each finch species present on an island had a beak size that matched a peak quite closely.
- The species present differed in beak size, so that only one species “occupied” a peak.
- The number of finch species present on an island increased with the number of distinct peaks in predicted carrying capacity.
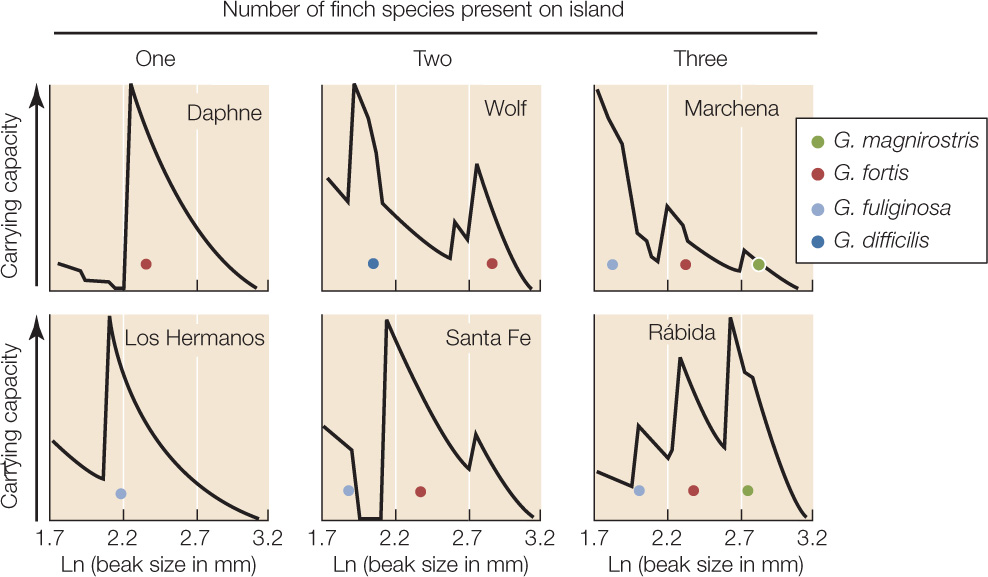 Figure 43.8: Intra- and Interspecific Competition Influence the Morphology of Coexisting Species In the Galápagos archipelago, seed-eating finches (Geospiza spp.) use their beaks to crack open seeds, which are often in short supply. Individuals with big beaks can crack large, hard seeds that individuals with small beaks cannot eat, whereas small-beaked birds gain more fitness than do large-beaked birds from eating small, soft seeds. Dolph Schluter and Peter Grant documented how the islands differed both in their seed resources and in the morphology of Geospiza species present.a
Figure 43.8: Intra- and Interspecific Competition Influence the Morphology of Coexisting Species In the Galápagos archipelago, seed-eating finches (Geospiza spp.) use their beaks to crack open seeds, which are often in short supply. Individuals with big beaks can crack large, hard seeds that individuals with small beaks cannot eat, whereas small-beaked birds gain more fitness than do large-beaked birds from eating small, soft seeds. Dolph Schluter and Peter Grant documented how the islands differed both in their seed resources and in the morphology of Geospiza species present.a
CONCLUSION
The availability of seeds of different sizes determines how many species of Geospiza occur on an island and what their beak sizes are.
ANALYZE THE DATA
- Explain how intraspecific competition could cause the average beak size of a hypothetical finch species that colonizes an island to evolve to match a peak in carrying capacity.
- Suppose the G. fuliginosa population on Marchena goes extinct, and the island is subsequently colonized by G. fuliginosa individuals from Rábida. Do you expect the average beak size of the colonizing population to increase or decrease through time? Explain your reasoning.
- Explain how interspecific competition could cause peaks in seed availability to have no more than one associated finch species.
aD. Schluter and P. R. Grant. 1984. American Naturalist 123: 175–196.
Imagine an island without finches that supports one abundant plant species producing seeds of a certain size and hardness. Suppose next that individuals of a finch species colonize the island. The new colonists will vary somewhat in beak size, which is related to body size—big birds have big, strong beaks. One beak (and body) size will be best: relative to this “optimal” size, smaller birds will have more difficulty cracking the seeds open, whereas larger birds will have to allocate more energy to maintenance of their larger bodies, thus leaving less for reproduction (see Concept 42.3). Individuals with beaks closest to the optimal size will leave the most offspring. As a consequence, the average beak size will evolve toward the optimum by stabilizing natural selection (see Figure 15.13A). Furthermore, the carrying capacity (see Concept 42.4)—the number of birds that can be supported by the available seed crop—will increase as birds that convert seeds into offspring most efficiently come to predominate in the population.
Interspecific competition can lead to resource partitioning and coexistence
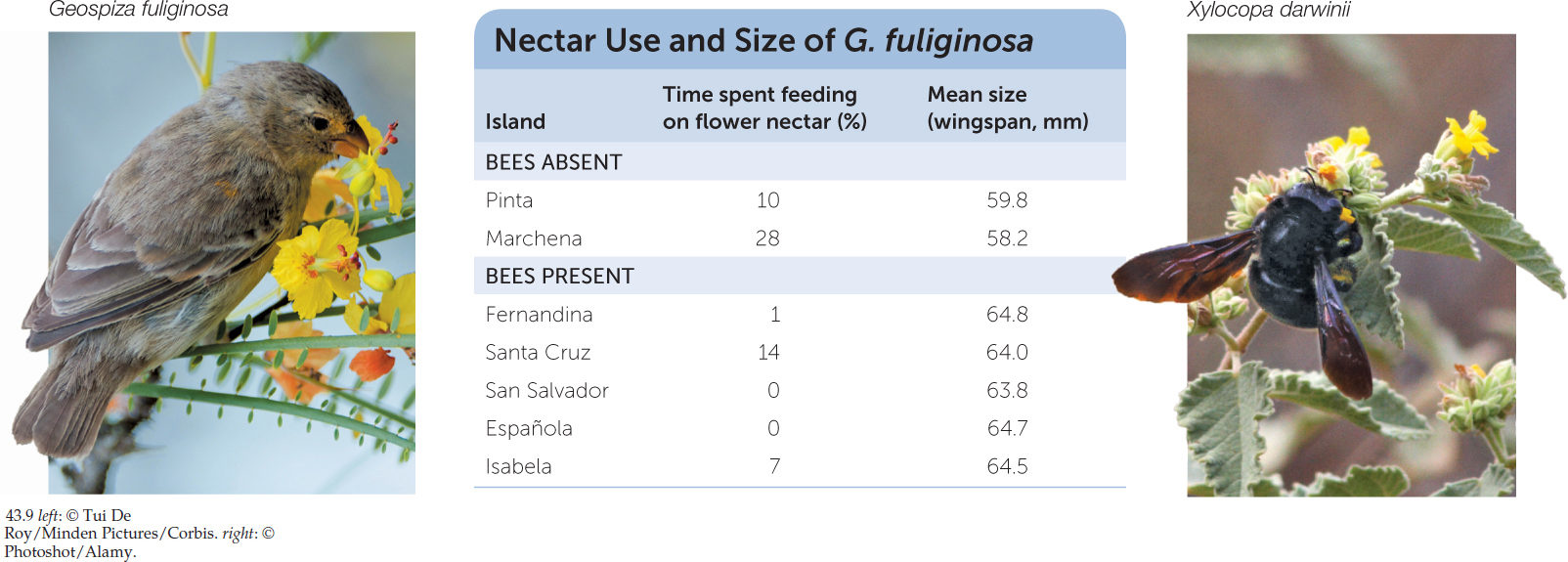
Interspecific competition, like intraspecific competition, can lead to evolution, as is illustrated by another interaction in the Galápagos archipelago, between a finch and an insect. The small ground finch (Geospiza fuliginosa) consumes flower nectar as well as seeds. Small individuals are better at obtaining nectar from the islands’ small flowers, but large individuals have larger beaks that are better at cracking seeds. Carpenter bees (Xylocopa darwinii) compete with G. fuliginosa for nectar on some islands but are absent from others. Small finches are affected more strongly by competition from bees than are larger individuals. As a consequence, the finches are larger in size, and drink less nectar, on islands where carpenter bees are present (FIGURE 43.9). This evolutionary shift means that resource use by G. fuliginosa has diverged from that of its bee competitors on islands where the two species coexist—an example of resource partitioning (see Concept 43.2) that resulted from natural selection.
Consumer–resource interactions can lead to an evolutionary arms race
Predators, parasites, and herbivores benefit if they can acquire lots of nutritious food quickly and inexpensively, whereas resource species benefit if they can evade or deter these natural enemies at little cost. The interests of consumer and resource species are obviously at odds. These opposing interests can lead to an “evolutionary arms race,” in which prey continually evolve better defenses, predators continually evolve better offenses, and neither gains any lasting advantage over the other. The Red Queen summed up this situation in Lewis Carroll’s Through the Looking-Glass with the words “It takes all the running you can do, to keep in the same place.”
893
894
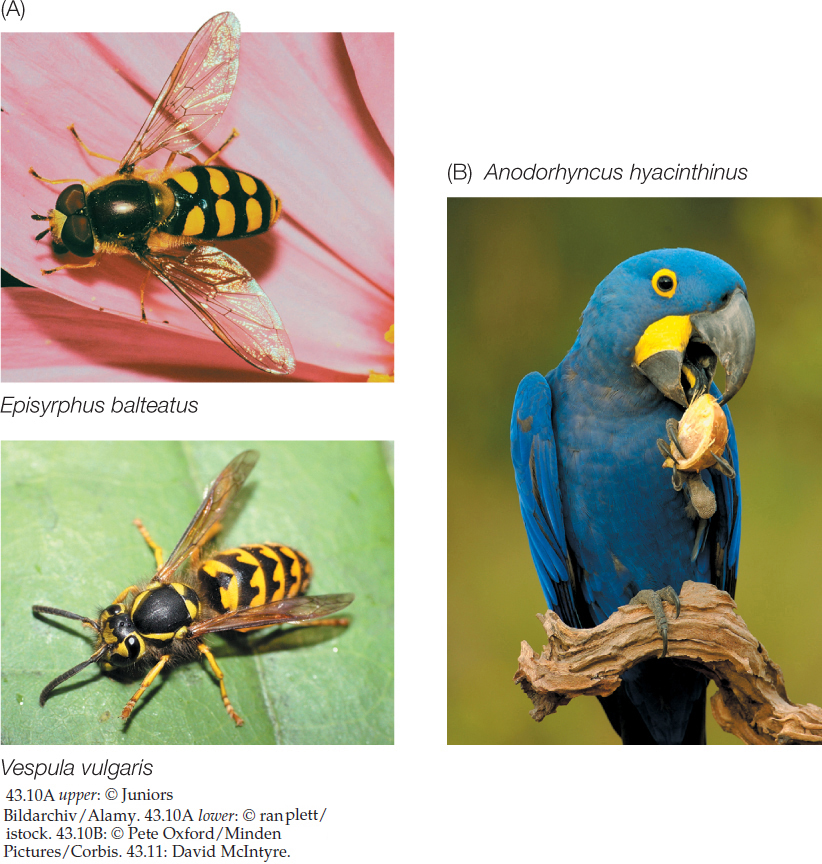
Resource species have various defensive strategies available to them. Some mobile animals use speed, size, or weapons to thwart predators. Others hide or use camouflage to avoid being detected, or mimic unpalatable species (FIGURE 43.10A). Immobile organisms have other tricks up their sleeves; for example, they may have evolved thick armor, or they may be poisonous or not nutritious. In response to these strategies, natural selection in populations of consumers favors greater speed, size, or strength; keen senses; armor-piercing or crushing tools (FIGURE 43.10B); or means of detoxifying poisons.
Plants produce a great variety of defensive chemicals, as we saw in Chapter 28. Used in small quantities, many of these chemicals, including the caffeine of coffee and tea, the mustard oils of cabbages, the capsaicins of chilis, and the piperine of black pepper, spice up our own lives. But the production of such compounds evolved because they are toxic or repellant to some herbivores and pathogens. Defensive compounds explain in part why the leaf-cutter ant Atta cephalotes harvests leaves from only 17 of 332 plant species that grow in the Florencia Norte forest of Costa Rica: the terpene-like compounds (see Table 28.1) found in the leaves of many of the plants make them toxic either to the ants or to their fungus.
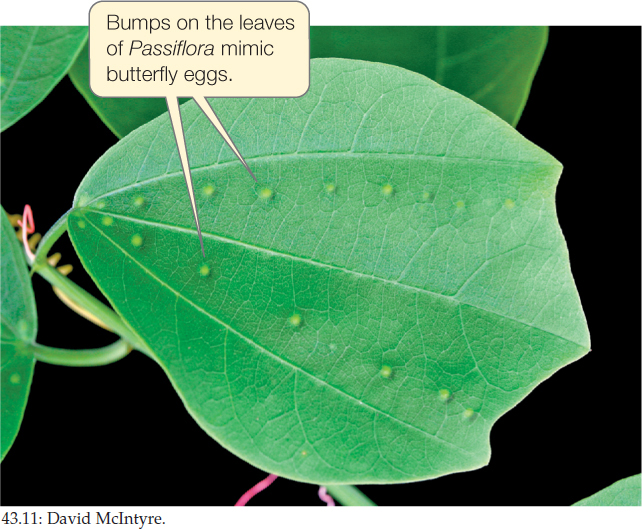
Many herbivores have evolved ways around plant chemical defenses. The caterpillars of Heliconius butterflies, for example, store or detoxify the cyanide-containing defensive compounds of the passionflower (Passiflora) vines they feed on, and even use these poisons as defenses against their own predators. Some Passiflora species, conversely, have evolved modified leaf structures that mimic butterfly eggs (FIGURE 43.11). Since female butterflies will not lay eggs on leaves that already carry eggs, these structures reduce the plant’s probability of being eaten by Heliconius caterpillars.
Mutualisms involve conflict of interest
Mutualisms are often misunderstood. We sometimes hear that “bees visit flowers to pollinate them,” or that “flowers provide food for their pollinators,” but such statements imply an impossible evolutionary process in which species exhibit traits that evolved to benefit another species. As Charles Darwin said about his theory of natural selection (see Concept 15.1),
895
If it could be proved that any part of the structure of any one species had been formed for the exclusive good of another species, it would annihilate my theory, for such could not have been produced through natural selection.
In truth, most pollinators visit flowers to get food and just happen to pollinate the flowers in the process; flowers provide food (usually as little as possible) to lure animals, not to fatten them up; birds and bees respond to any conspicuous color that signals the presence of food; and most flowers are visited by any animal that gains economically by doing so, whether it pollinates the plant or not. In the rough-and-tumble world of ecological interactions, species benefit other species not out of altruism but because acting in their own self-interest happens to benefit others.
LINK
The coevolution of plants and pollinators is discussed in Concept 21.5
Interactions within and among species can result in evolution
Crabs feed on mussels, and individual young mussels can sometimes detect the presence of crabs and respond by developing a thicker protective shell as they grow to adult size. The abilities to detect and to respond to crabs are inherited traits.
On the Atlantic coast of North America, blue mussels (Mytilus edulis) face two non-native crab predators. The European green crab (Carcinus maenas) was introduced to the United States almost 200 years ago, and the Asian shore crab (Hemigrapsus sanguineus) arrived only 20 years ago. C. maenas has spread all along the Atlantic coast of North America, whereas H. sanguineus has not yet reached northern Maine. Have mussels evolved the ability to detect this new enemy and respond to it?
Researchers collected very young M. edulis from northern populations, which have experienced predation by C. maenas but not by H. sanguineus, and from southern populations, which have experienced both predator species. They grew mussels from each population on floating docks under three different conditions: no crab nearby (controls); a hungry C. maenas caged nearby; or a hungry H. sanguineus caged nearby. After 3 months, researchers measured mussel shell thickness.a
Use the graph to answer the following questions. Error bars indicate 95% confidence intervals of mean shell thickness; bars with different letters are statistically different from one another (P < 0.05). See Appendix B for discussion of statistical concepts.
- Have mussel populations exposed to predation by nonnative crabs evolved the ability to detect and respond to them by thickening their shells? Explain your answer.
- Which comparison is critical to your conclusion?
- Do mussels in southern populations have thinner shells than mussels in northern populations? Would a difference in average shell thickness between the two populations invalidate your conclusion? Why or why not?
- What do these data suggest about how quickly evolution can take place?
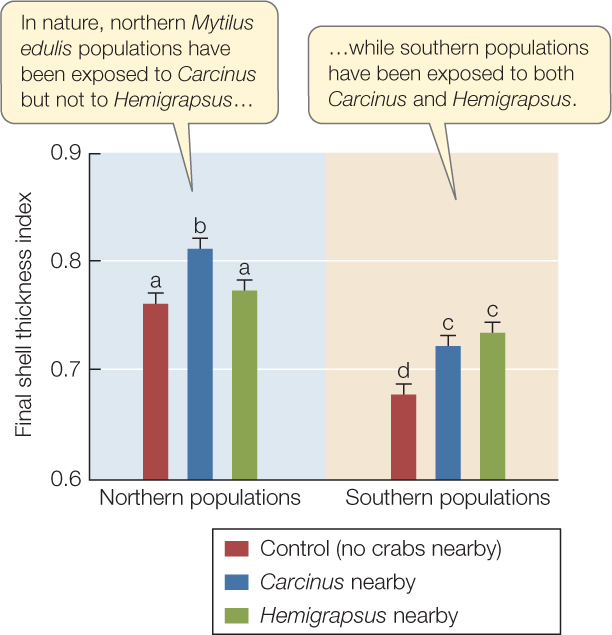
aA. S. Freeman and J. E. Byers. 2006. Science 313: 831–833.
896
All mutualisms involve “biological barter”: the exchange of resources and services. Plants and pollinators exchange food (usually nectar or pollen) for pollen transport. Plants and frugivores exchange food (fruit) for seed dispersal. Some mutualists (such as ants and plants) exchange defense or parasite and pathogen control for food or shelter. Others (such as plants and mycorrhizae, or mammals and gut bacteria) exchange one kind of nutrient for another. In such “barter” systems, each species is selected to obtain the greatest benefit for the least cost. Therefore the net effect of the mutualism on fitness may vary depending on environmental conditions that influence the value and cost of the goods and services that are exchanged. Mycorrhizae, for example, benefit plants in nutrient-poor soils, but they can be a liability to plants in nutrient-rich soils, where the cost of feeding the mycorrhizae outweighs their value in nutrient uptake. Accordingly, plants in nutrient-rich soils excrete fewer carbohydrate-rich compounds from their roots, thus discouraging colonization by mycorrhizal fungi.
Because mutualisms are diverse and often complex, it is difficult to predict their consequences for the evolution of the species involved. Some mutualisms evolve into one-on-one relationships with ever-tighter reciprocal adaptation—leaf-cutter ants and fungus are a case in point. Others evolve into many-species relationships that rely on generalized adaptations; many mutualisms between plants and the animals that disperse their seeds fall into this category. Some mutualisms are unstable and dissolve into consumer–resource relationships. Understanding the conditions under which mutualisms follow different evolutionary trajectories is an active area of current research.
CHECKpointCONCEPT43.4
- What goods or services are exchanged in the “biological barter” between leaf-cutter ants and their fungus?
- Why might individual plants in populations within the geographic range of an insect herbivore contain high levels of defensive chemicals while individuals in populations outside the insect’s range produce no defensive chemicals?
- We claimed that pollinators do not visit flowers “to pollinate them,” but females of some yucca moth species carefully collect pollen, carry it to the next flower they visit, and place it on the stigma before depositing eggs in the flower’s ovary. The eggs will hatch into larvae that eat some developing seeds. Does this behavior “annihilate” Darwin’s theory of natural selection? Explain why or why not.
The ecological and evolutionary effects of interactions among species determine which species can coexist in an area and what their abundances are. The number of species present, their phenotypic attributes, and their relative abundances determine the structure and function of communities—the topic of the next chapter.
How could the intricate ecological relationship between leaf-cutter ants and fungus have evolved?
ANSWER We have seen that interactions between species can be mutually beneficial, positive for one species and negative or neutral for the other, negative for one species and neutral for the other, or mutually detrimental (Concept 43.1). These interspecific interactions affect the fitness of the individuals involved (Concept 43.1), and any traits that increase the benefit of a positive interaction, or decrease the cost of a negative interaction, will therefore spread by natural selection (Concept 43.4).
Although more details remain to be discovered, we now can outline a scenario for the evolution of the remarkable ecological interaction between leaf-cutter ants and their cultivated fungus. Phylogenetic analysis (see Chapter 16) suggests that fungus cultivation originated when ancestors of the leaf-cutters began to feed on fungi growing on the refuse in their nests. Ant colonies that provided better growing conditions for these fungi—by providing more refuse and, eventually, by providing fresh leaf material (which the ants cannot themselves digest)—and that chose productive fungus varieties to propagate (by moving fungal masses into newly founded nests and into newly dug chambers in established nests)—had more food to eat, and thus higher fitness, than colonies that did not do these things (FIGURE 43.12). Thus fungus-tending behaviors evolved by natural selection. Conversely, fungi that provided better ant food benefited from faster ant colony growth and propagation, and these fungal adaptations also evolved by natural selection.
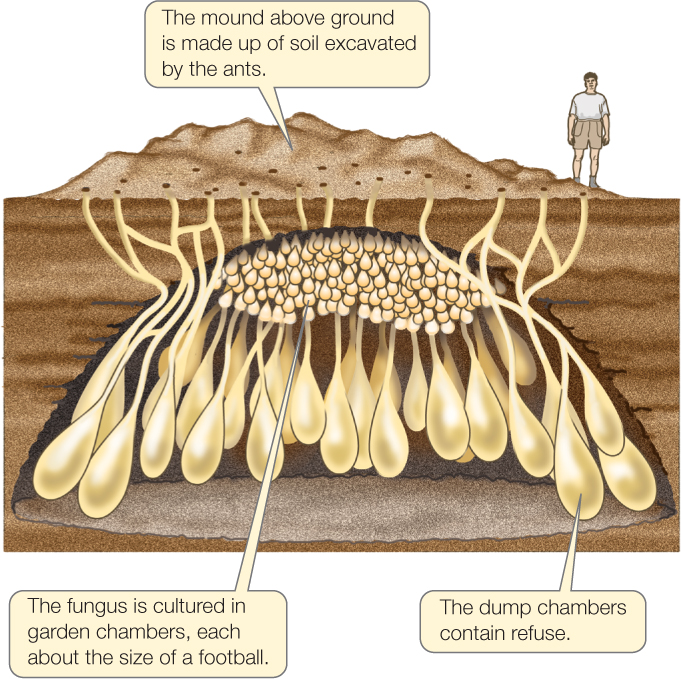
897
This history of reciprocal adaptation led to the great ecological success of leaf-cutter ants and their fungus. Leaf-cutter ants are not only the most important herbivores in the Neotropics, but also have expanded into dry environments, such as the Arizona desert, that are normally hostile to fungi.