CONCEPT45.2 Biological, Geological, and Chemical Processes Move Materials through Ecosystems
The sun provides a steady input of radiant energy to Earth. A small amount of this radiant energy is captured by photosynthetic organisms and converted into chemical energy, as we saw in Concept 44.3. This energy fuels the metabolism of all organisms in the community and is ultimately transformed into waste heat. In contrast, the chemical elements that make up organisms come from within the Earth system itself. There is essentially a fixed amount of each element because little matter escapes Earth’s gravity or enters the Earth system from space. Whereas Earth is an open system with respect to energy, it is a closed system with respect to matter. This does not mean that the distribution of matter is static, however: energy from the sun and heat from Earth’s interior drive biological, geological, and chemical processes that transform matter and move it around the global ecosystem.
The forms and locations of elements determine their accessibility to organisms
Imagine an atom. At any time, this atom occurs in a particular molecular form and occupies a particular location in Earth’s closed system. It might be part of a living organism or of dead organic matter. It might be part of a molecule in the atmosphere, in a body of fresh or salt water, or in soil, sediment, or rock. If in rock, it might be part of the surface crust of Earth or more deeply buried. These alternative forms and locations of matter can be thought of as compartments. Atoms cycle repeatedly among compartments when they are chemically transformed or physically moved. The atoms in Earth’s crust, for example, have gone through repeated cycles of being buried, compressed, heated, uplifted, eroded, and deposited again in sediments (see Figure 18.2). The compartments of the global ecosystem, and the processes that cycle materials among them, can be depicted in a systems diagram (FIGURE 45.5; see also Figure 1.6).
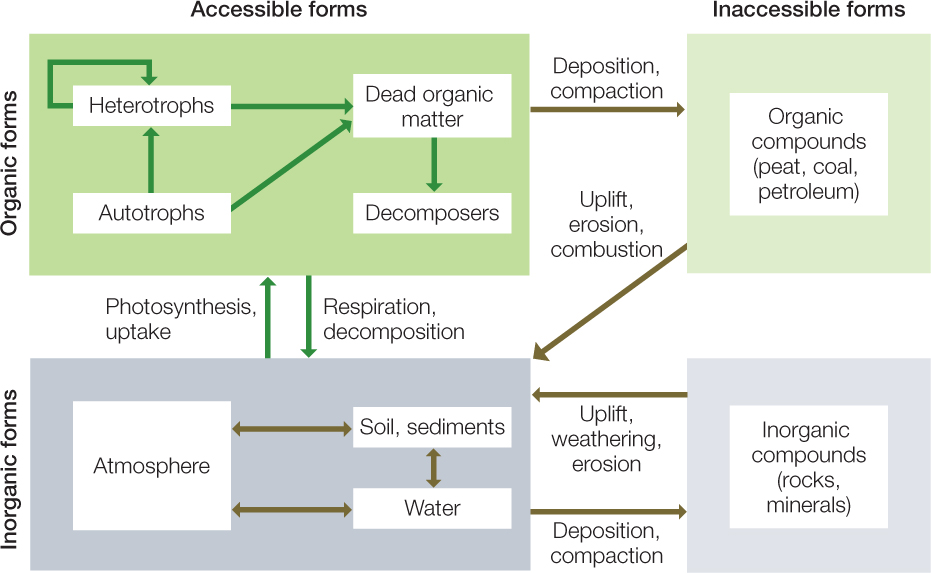
The chemical form in which an atom exists determines whether it is accessible to life. With rare exceptions, autotrophs take up carbon in the form of CO2, and they absorb nutrients such as nitrogen, phosphorus, and potassium in the form of ions dissolved in water (see Concept 25.1). Heterotrophs (including decomposers) extract most of the materials they need from living or dead organic matter. But even when an atom is in the right chemical form, organisms cannot use it if it is in the wrong place. Matter that is buried too deeply, or that occurs in places that are too hot, too cold, or otherwise too hostile, is inaccessible to life.
The processes that supply matter to organisms occur in the biosphere, the thin skin at Earth’s surface where atmosphere, land, and water are in contact with one another and where organisms live. The biosphere is only about 23 kilometers thick—a mere 1/277 of Earth’s radius—extending up to the stratosphere and down to the abyssal zone of the oceans.
Movement of matter is driven by biogeochemical processes
Matter is moved among the compartments of the global ecosystem by processes that are biological, geological, and chemical; thus these movements are called biogeochemical cycles. Biological and abiotic chemical reactions combine elements with other elements and compounds, converting them between inorganic and organic forms or between oxidized and reduced states. Many of these reactions involve the abundant elements oxygen and hydrogen. Chemical reactions are also involved in the weathering of rock during soil formation. Physical processes—the circulation of air and water and convective flows within Earth’s mantle—move matter through the Earth system, alternately exposing it to air, water, and solar radiation or to heat and pressure.
LINK
Oxidation–reduction (“redox”) reactions are described in Concept 6.1, weathering and soil formation in Concept 25.1, plate tectonic processes in Concept 18.2, and circulation of the atmosphere and oceans in Concept 41.2
921
The total amount, or pool, of an element or molecule in a given compartment depends on its rate of movement into and out of that compartment. The rate of movement, or flux, from one compartment to another is measured in amount per unit of time. If the fluxes into and out of a compartment are not identical, the pool will grow or shrink. Oxidation of organic matter by respiration, wildfires, or fossil fuel combustion, for example, will increase the atmospheric pool of carbon unless photosynthesis and other biogeochemical processes remove carbon from the atmosphere at an identical or greater rate.
All of the materials contained in the bodies of living organisms are ultimately derived from abiotic sources, either atmospheric gases or materials dissolved in water. Primary producers (see Concept 44.3) take up elements from these inorganic compartments, accumulating them as biomass. Trophic interactions (see Concept 43.3) pass those elements on to heterotrophs, which also take in other inorganic materials such as oxygen and water as part of their metabolism. Eventually all of these materials enter the nonliving organic matter compartment. Decomposers break down the dead organic matter into simpler chemical compounds and elements that can be taken up by primary producers. Much of this recycling of materials occurs locally, but some occurs on a larger scale when materials diffuse away in gaseous form or are carried out of the local ecosystem by wind or water.
CHECKpointCONCEPT45.2
- What is the distinction between the concepts of pool and flux with regard to a given chemical element or compound?
- Explain the meaning of “closed system” in the statement that Earth is a closed system with respect to matter.
- Draw a systems diagram to show how energy from sunlight enters and flows through the global ecosystem. (Hint: See Concept 6.5 and Figures 44.6 and 45.5.)
We have now seen that the materials that make up living tissue—chemical elements and compounds—cycle through ecosystems. Let’s next consider the cycles of three materials that are especially critical for ecosystem function.