9-1 Earth’s atmosphere, oceans, and surface are extraordinarily active
The crew of an alien spacecraft exploring our solar system might overlook Earth altogether. Although it is the largest of the terrestrial planets, with a mass greater than that of Mercury, Venus, and Mars put together, Earth is far smaller than any of the giant Jovian planets (see Table 7-1 and Table 9-1). But a more careful inspection would reveal that Earth is unique among all the planets that orbit the Sun.
TABLE 9-1 EARTH DATA

Dynamic Earth: Water, Air, and Land
To understand our dynamic planet, we must understand the energy sources that power its activity
Unlike the arid surfaces of Venus and Mars, Earth’s surface is very wet. Indeed, nearly 71% of Earth’s surface is covered with water (Figure 9-1). Water is also locked up into many of the minerals that make Earth’s rocks, including those found in the driest deserts. Furthermore, Earth’s liquid water is in constant motion. The oceans ebb and flow with the tide, streams and rivers flow down to the sea, and storms whip lake waters into a frenzy.

Earth’s Dynamic Atmosphere This space shuttle image shows thunderstorm clouds over the African nation of Zaire. The tops of thunderstorm clouds frequently reach altitudes of 10,000 m (33,000 ft) or higher. At any given time, nearly 2000 thunderstorms are in progress over Earth’s surface.
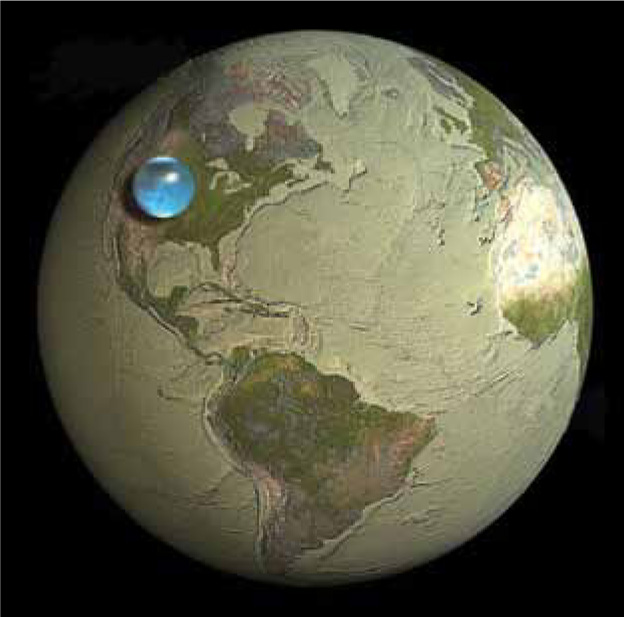
Earth’s Dynamic Oceans This image shows the relative size of Earth compared to a sphere containing all of Earth’s water. The sphere is about 860 miles in diameter and includes fresh water, oceans, ice, and even water in the atmosphere. Nearly three-quarters of Earth’s surface is covered with water, a substance that is essential to the existence of life. In contrast, there is no liquid water at all on Mercury, Venus, Mars, or the Moon.
Earth’s atmosphere is also in a state of perpetual activity. Winds blow at all altitudes, with speeds and directions that change from one hour to the next. The atmosphere is at its most dynamic when water evaporates to form clouds, then returns to the surface as rain or snow (Figure 9-2). While other worlds of the solar system have atmospheres, only Earth’s contains the oxygen that animals (including humans) need to live.
The combined effects of water and wind cause erosion of mountains and beaches. But these are by no means the only forces reshaping the face of our planet. All across Earth we find evidence that the surface has been twisted, deformed, and folded (Figure 9-3). What is more, new material is continually being added to Earth’s surface as lava pours forth from volcanoes and from immense cracks in the ocean floors. At the same time, other geologic activity slowly buries old surface material, or pulls material back into our planet’s interior. These processes and others work together to renew and refresh our planet’s exterior. Thus, although Earth is some 4.54 billion (4.54 × 109) years old, much of its surface is less than 200 million years old (Figure 9-4). (We saw in Section 8-2 how scientists use radioactive dating to determine the ages of rocks and of Earth as a whole.)

Old and Young Rocks in the Grand Canyon The ages of rocks in Arizona’s Grand Canyon demonstrate that geologic processes take place over very long time scales.

Earth’s Dynamic Surface These tan-colored ridges, or hogbacks, in Colorado’s Rocky Mountains were once layers of sediment at the bottom of an ancient body of water. Forces within Earth folded this terrain and rotated the layers into a vertical orientation. The layers were revealed when wind and rain eroded away the surrounding material.
Solar Energy and Convection
 What powers all of this activity in Earth’s oceans, atmosphere, and surface? There are three energy sources: radiation from the Sun, the tidal effects of the Moon and Sun, and Earth’s own internal heat (Table 9-2).
What powers all of this activity in Earth’s oceans, atmosphere, and surface? There are three energy sources: radiation from the Sun, the tidal effects of the Moon and Sun, and Earth’s own internal heat (Table 9-2).
| Activity | Energy Sources |
|---|---|
| Motion of water in oceans, lakes, rivers | Solar energy, tidal forces |
| Motion of the atmosphere | Solar energy |
| Reshaping of surface | Earth’s internal heat |
| Life | Solar energy (a few species that live on the ocean floor make use of Earth’s internal heat) |
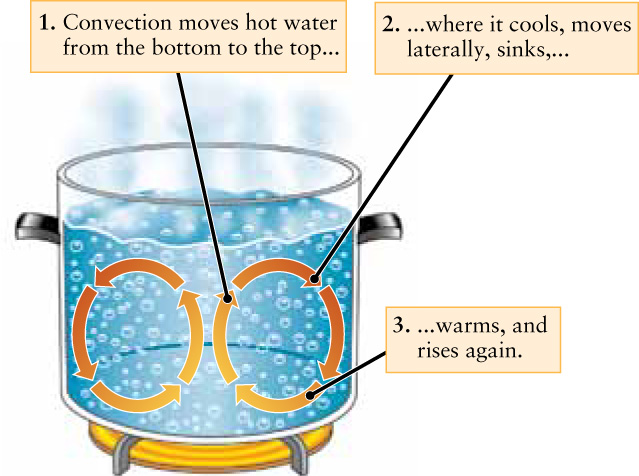
The Sun is the principal source of energy for the atmosphere. Earth’s surface is warmed by sunlight, which in turn warms the air next to the surface. Hot air is less dense than cool air and so it tends to rise (see the discussion of density in Box 7-1). As the air rises, it transfers heat to its surroundings. As a result, the rising air cools and becomes denser. It then sinks downward to be heated again, and the process starts over. This up-and-down motion is called convection, and the overall pattern of circulation is called a convection current. (You can see convection currents in action by heating water on a stove, as Figure 9-5 shows.) Convection currents also play a key role in reshaping Earth’s surface as warmer low-density material rises up from Earth’s interior, cools and increases its density, and then sinks back down.
Solar energy also powers atmospheric activity by evaporating water from the surface. The energy in the water vapor is released later when it condenses to form water droplets, like those that make up clouds. In a typical thunderstorm (see Figure 9-2), the amount of energy released when this water condenses is as much as a city of 100,000 people uses in a month!
Solar energy also helps to power the oceans. Warm water from near the equator moves toward the poles, while cold polar water returns toward the equator. As we saw in Section 4-8, however, there is back-and-forth motion of the oceans due to the tidal forces of the Moon and Sun. Sometimes these two influences can reinforce each other, as when a storm (caused by solar energy) reaches a coastline at high tide (caused by tidal forces), producing waves strong enough to seriously erode beaches and sea cliffs.
Neither solar energy nor tidal forces can explain the reshaping of Earth’s surface suggested by Figure 9-3. Rather, most geologic activity is powered by heat flowing from the interior of Earth itself. Most of the heat is caused by the nuclear decay of radioactive elements such as uranium and thorium deep inside Earth. There is even some heat still working its way out from the energetic collisions among planetesimals that created Earth (see Section 8-5).
The heat flow from Earth’s interior to its surface is minuscule—just 1/6000 as great as the flow of energy we receive from the Sun—but it has a profound effect on the face of our planet. In Section 9-2 and Section 9-3 we will explore the interior structure of Earth and discover that the heat-driven convection illustrated in Figure 9-5 also takes place in our Earth.
CONCEPT CHECK 9-1
Consider the convection illustrated in Figure 9-5. Is gravity necessary for convection to occur? Can convection occur if the temperature is the same above and below the fluid?
For convection to occur, gravity is necessary, as well as a cooler temperature on top of the fluid. Convection depends on lower-density material rising when surrounded by higher-density material, and this requires gravity. But, the density depends on temperature, and if the entire fluid were at the same temperature, there would be no changes in fluid density; thus, a cooler temperature on top is needed.
Earth’s Surface Temperature and the Greenhouse Effect
Since so little heat comes from inside our planet, the average surface temperature of Earth depends almost entirely on the amount of energy that reaches us from the Sun in the form of visible light and other wavelengths of electromagnetic radiation. (In an analogous way, whether you feel warm or cool outdoors on a summer day depends on whether you are in the sunlight, receiving lots of solar energy, or in the shade, receiving little of this energy.)
If Earth did nothing but absorb radiation from the Sun, it would get hotter and hotter until the surface temperature became high enough to melt rock. Happily, there are two reasons why this does not happen. One is that the clouds, snow, ice, and sand reflect about 31% of the incoming sunlight back into space. The fraction of incoming sunlight that a planet reflects is called its albedo (from the Latin for “whiteness”); thus, Earth’s albedo is about 0.31. As a result, only 69% of the incoming solar energy is absorbed by Earth. A second reason is that Earth also emits radiation into space because of its temperature, in accordance with the laws that describe heated dense objects (see Section 5-3). Earth’s average surface temperature is nearly constant, which means that on the whole it is neither gaining nor losing energy. Thus, the rate at which Earth emits energy into space must equal the rate at which it absorbs energy from the Sun.
To better understand this balance between absorbed and emitted radiation, remember that Wien’s law tells us that the wavelength at which such an object emits most strongly (λmax) is inversely proportional to its temperature (T) on the Kelvin scale (see Section 5-4 and Box 5-2). For example, the Sun’s surface temperature is about 5800 K, and sunlight has its greatest intensity at a wavelength λmax of 500 nm, which is green light in the middle of the visible spectrum. Earth’s average surface temperature of 287 K is far lower than the Sun’s, so Earth radiates most strongly at longer wavelengths in the infrared portion of the electromagnetic spectrum. The Stefan-Boltzmann law tells us that temperature also determines the amount of radiation that Earth emits: The higher the temperature, the more energy it radiates.
Given the amount of energy reaching us from the Sun each second as well as Earth’s albedo, we can calculate the amount of solar energy that Earth should absorb each second. Since this amount must equal the amount of electromagnetic energy that Earth emits each second, which in turn depends on Earth’s average surface temperature, we can calculate what Earth’s average surface temperature should be. The result is a very chilly 254 K (-19°C = -2°F), so cold that oceans and lakes around the world should be frozen over. In fact, Earth’s actual average surface temperature is 287 K (14°C = 57°F). What is wrong with our model? Why is Earth warmer than we would expect?
The explanation for this discrepancy is called the greenhouse effect: Our atmosphere prevents some of the radiation emitted by Earth’s surface from escaping into space. Certain gases in our atmosphere called greenhouse gases, among them water vapor (H2O) and carbon dioxide (CO2), are transparent to visible light but not to infrared radiation. Consequently, visible sunlight has no trouble entering our atmosphere and warming the surface. But the infrared radiation coming from the heated surface is partially trapped by the atmosphere, thus raising the temperatures of both the atmosphere and the surface. As the surface and atmosphere become hotter, they both emit more infrared radiation, part of which is able to escape into space. The temperature levels off when the amount of infrared energy that escapes just balances the amount of solar energy reaching the surface (Figure 9-6). The result is that our planet’s surface is some 33°C (59°F) warmer than it would be without the greenhouse effect, and water remains unfrozen over most of Earth.
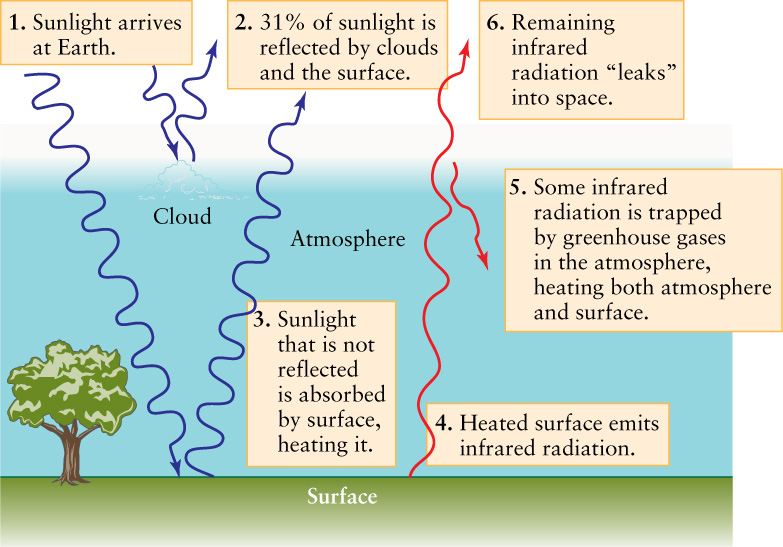
 The Greenhouse Effect Sunlight warms Earth’s surface, which due to its temperature emits infrared radiation. Much of this radiation is absorbed by atmospheric water vapor and carbon dioxide, helping to raise the average temperature of the surface. Some infrared radiation does penetrate the atmosphere and leaks into space. In a state of equilibrium, the rate at which Earth loses energy to space in this way is equal to the rate at which it absorbs energy from the Sun.
The Greenhouse Effect Sunlight warms Earth’s surface, which due to its temperature emits infrared radiation. Much of this radiation is absorbed by atmospheric water vapor and carbon dioxide, helping to raise the average temperature of the surface. Some infrared radiation does penetrate the atmosphere and leaks into space. In a state of equilibrium, the rate at which Earth loses energy to space in this way is equal to the rate at which it absorbs energy from the Sun.
The warming caused by the greenhouse effect gives our planet the moderate temperatures needed for the existence of life. For more than a century, however, our technological civilizations have been adding greenhouse gases to the atmosphere at an unprecedented rate. As we will see in Section 9-7, the likely consequences to our climate are extremely grave.
CONCEPT CHECK 9-2
What is the positive impact that the greenhouse effect has on our planet?
By trapping heat, the greenhouse effect keeps Earth’s temperature warmer than it would otherwise be, allowing liquid water and hence life to exist on Earth.