23.5Carbon Atoms of Degraded Amino Acids Emerge as Major Metabolic Intermediates
Carbon Atoms of Degraded Amino Acids Emerge as Major Metabolic Intermediates
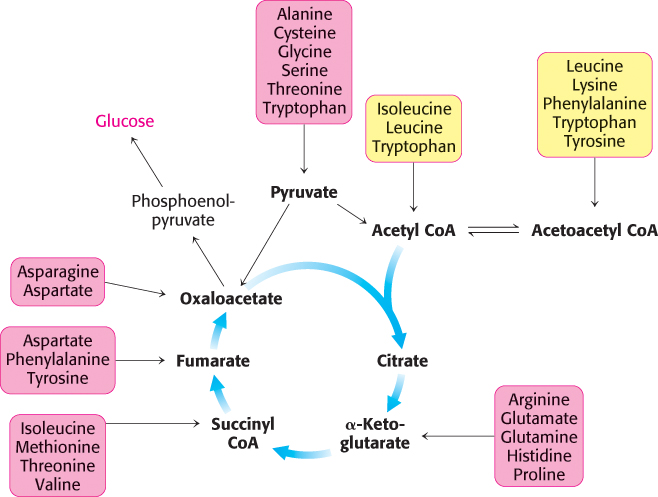
We now turn to the fates of the carbon skeletons of amino acids after the removal of the α-amino group. The strategy of amino acid degradation is to transform the carbon skeletons into major metabolic intermediates that can be converted into glucose or oxidized by the citric acid cycle. The conversion pathways range from extremely simple to quite complex. The carbon skeletons of the diverse set of 20 fundamental amino acids are funneled into only seven molecules: pyruvate, acetyl CoA, acetoacetyl CoA, α-ketoglutarate, succinyl CoA, fumarate, and oxaloacetate. We see here an example of the remarkable economy of metabolic conversions.
Amino acids that are degraded to acetyl CoA or acetoacetyl CoA are termed ketogenic amino acids because they can give rise to ketone bodies or fatty acids. Amino acids that are degraded to pyruvate, α-ketoglutarate, succinyl CoA, fumarate, or oxaloacetate are termed glucogenic amino acids. Oxaloacetate, generated from pyruvate and other citric acid cycle intermediates, can be converted into phosphoenolpyruvate and then into glucose (Section 16.3). Recall that mammals lack a pathway for the net synthesis of glucose from acetyl CoA or acetoacetyl CoA.
699
Of the basic set of 20 amino acids, only leucine and lysine are solely ketogenic (Figure 23.20). Isoleucine, phenylalanine, tryptophan, and tyrosine are both ketogenic and glucogenic. Some of their carbon atoms emerge in acetyl CoA or acetoacetyl CoA, whereas others appear in potential precursors of glucose. The other 14 amino acids are classed as solely glucogenic. We will identify the degradation pathways by the entry point into metabolism.
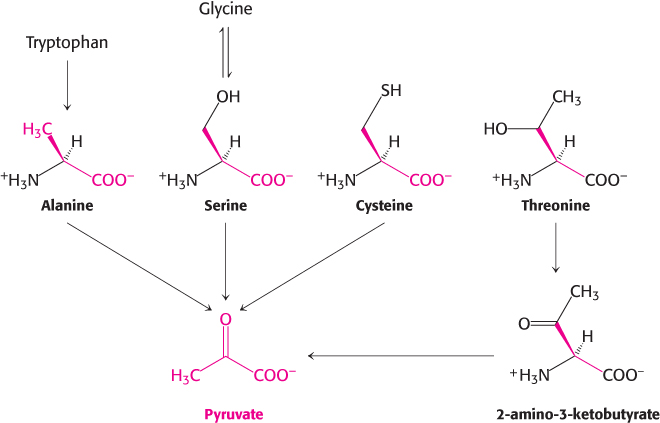
Pyruvate is an entry point into metabolism for a number of amino acids
Pyruvate is the entry point of the three-

700
As mentioned earlier in the chapter, glutamate is then oxidatively deaminated, yielding NH4+ and regenerating α-ketoglutarate. The sum of these reactions is
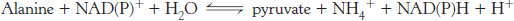
Another simple reaction in the degradation of amino acids is the deamination of serine to pyruvate by serine dehydratase.
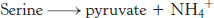
Cysteine can be converted into pyruvate by several pathways, with its sulfur atom emerging in H2S, SCN−, or SO32−.
The carbon atoms of three other amino acids can be converted into pyruvate. Glycine can be converted into serine by the enzymatic addition of a hydroxymethyl group or it can be cleaved to give CO2, NH4+, and an activated one-
Oxaloacetate is an entry point into metabolism for aspartate and asparagine
Aspartate and asparagine are converted into oxaloacetate, a citric acid cycle intermediate. Aspartate, a four-

Asparagine is hydrolyzed by asparaginase to NH4+ and aspartate, which is then transaminated.
Recall that aspartate can also be converted into fumarate by the urea cycle (Figure 23.16). Fumarate is a point of entry for half the carbon atoms of tyrosine and phenylalanine, as will be discussed shortly.
Alpha-ketoglutarate is an entry point into metabolism for five-carbon amino acids
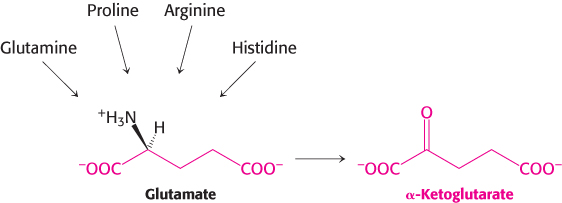
The carbon skeletons of several five-
Histidine is converted into 4-
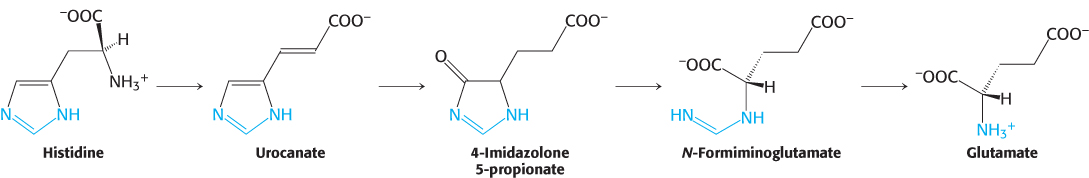
701
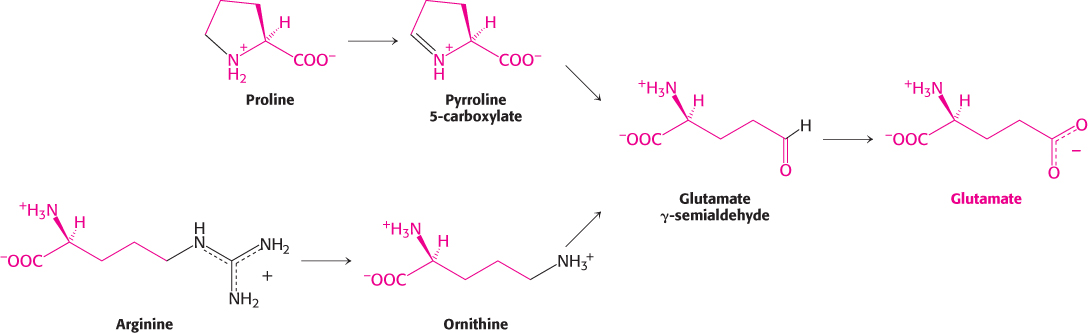
Glutamine is hydrolyzed to glutamate and NH4+ by glutaminase. Proline and arginine are each converted into glutamate γ-semialdehyde, which is then oxidized to glutamate (Figure 23.24).
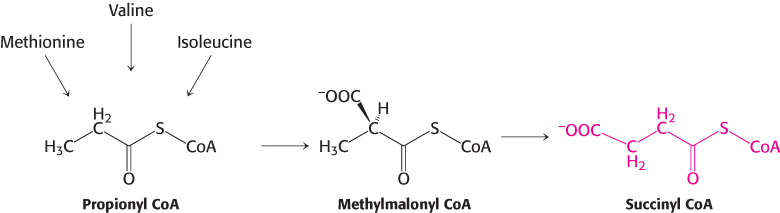
Succinyl coenzyme A is a point of entry for several nonpolar amino acids
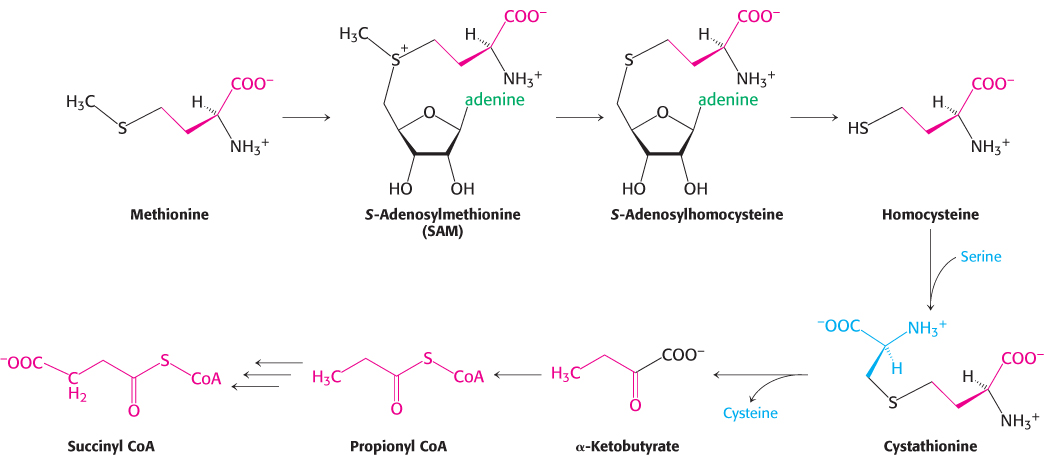
Succinyl CoA is a point of entry for some of the carbon atoms of methionine, isoleucine, and valine. Propionyl CoA and then methylmalonyl CoA are intermediates in the breakdown of these three nonpolar amino acids (Figure 23.25). This pathway from propionyl CoA to succinyl CoA is also used in the oxidation of fatty acids that have an odd number of carbon atoms. The mechanism for the interconversion of propionyl CoA and methylmalonyl CoA was presented in Section 22.3.
Methionine degradation requires the formation of a key methyl donor, S-adenosylmethionine
Methionine is converted into succinyl CoA in nine steps (Figure 23.26). The first step is the adenylation of methionine to form S-
The branched-chain amino acids yield acetyl CoA, acetoacetate, or propionyl CoA
The branched-
702
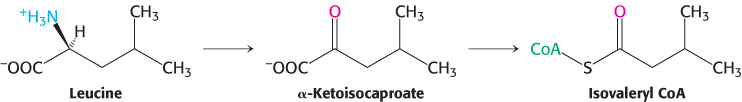
The α-ketoacids of valine and isoleucine, the other two branched-
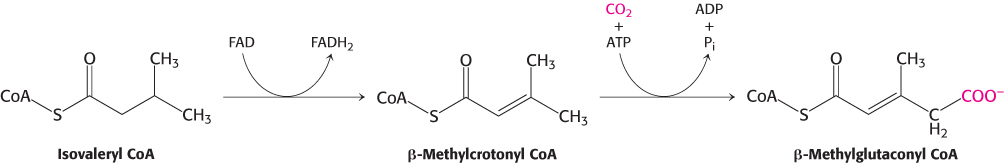
The isovaleryl CoA derived from leucine is dehydrogenated to yield β-methylcrotonyl CoA. This oxidation is catalyzed by isovalerylCoA dehydrogenase. The hydrogen acceptor is FAD, as in the analogous reaction in fatty acid oxidation that is catalyzed by acyl CoA dehydrogenase. β-Methylglutaconyl CoA is then formed by the carboxylation of β-methylcrotonyl CoA at the expense of the hydrolysis of a molecule of ATP. As might be expected, the carboxylation mechanism of β-methylcrotonyl CoA carboxylase is similar to that of pyruvate carboxylase and acetyl CoA carboxylase.
β-Methylglutaconyl CoA is then hydrated to form 3-
703
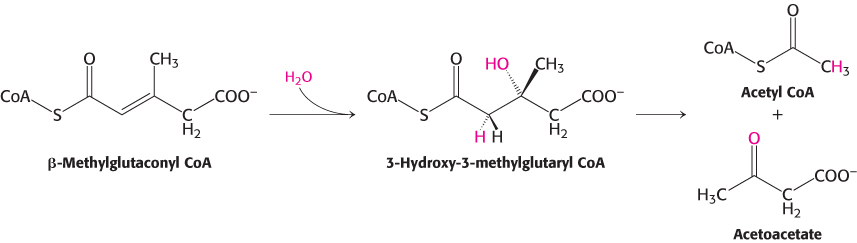
The degradative pathways of valine and isoleucine resemble that of leucine. After transamination and oxidative decarboxylation to yield a CoA derivative, the subsequent reactions are like those of fatty acid oxidation. Isoleucine yields acetyl CoA and propionyl CoA, whereas valine yields CO2 and propionyl CoA. The degradation of leucine, valine, and isoleucine validate a point made earlier (Chapter 15): the number of reactions in metabolism is large, but the number of kinds of reactions is relatively small. The degradation of leucine, valine, and isoleucine provides a striking illustration of the underlying simplicity and elegance of metabolism.
Oxygenases are required for the degradation of aromatic amino acids
The degradation of the aromatic amino acids yields the common intermediates acetoacetate, fumarate, and pyruvate. The degradation pathway is not as straightforward as that of the amino acids previously discussed. For the aromatic amino acids, molecular oxygen is used to break the aromatic ring.
The degradation of phenylalanine begins with its hydroxylation to tyrosine, a reaction catalyzed by phenylalanine hydroxylase. This enzyme is called a monooxygenase (or mixed-
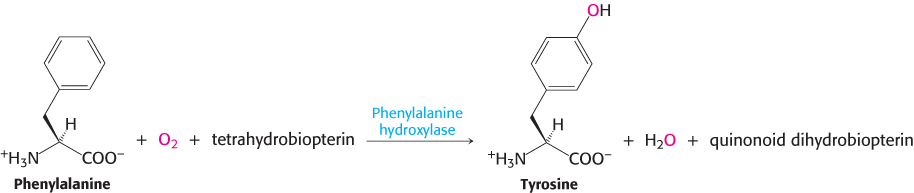
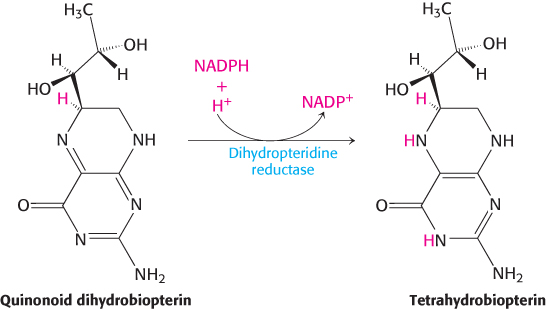
The reductant here is tetrahydrobiopterin, an electron carrier that has not been previously discussed and is derived from the cofactor biopterin. Because biopterin is synthesized in the body, it is not a vitamin. The quinonoid form of dihydrobiopterin is produced in the hydroxylation of phenylalanine. It is reduced back to tetrahydrobiopterin by NADPH in a reaction catalyzed by dihydropteridine reductase.
704
The sum of the reactions catalyzed by phenylalanine hydroxylase and dihydropteridine reductase is
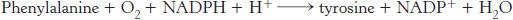
Note that these reactions can also be used to synthesize tyrosine from phenylalanine.
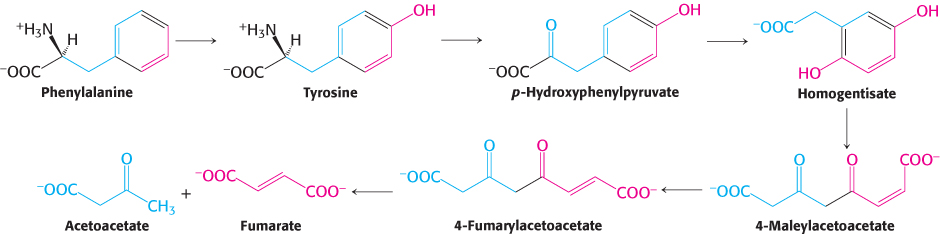
The next step in the degradation of phenylalanine and tyrosine is the transamination of tyrosine to p-
Tryptophan degradation requires several oxygenases (Figure 23.28). Tryptophan 2,3-
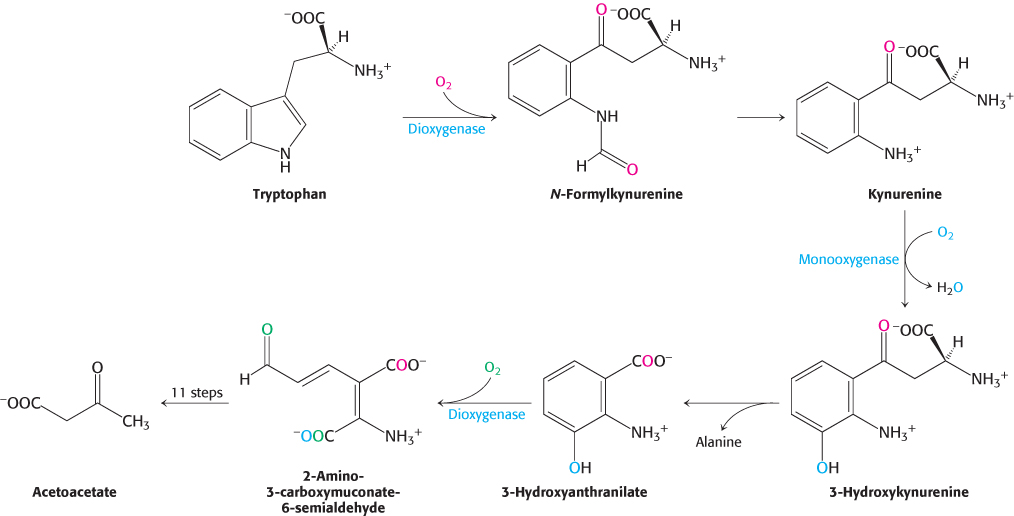
705