24.3Feedback Inhibition Regulates Amino Acid Biosynthesis
Feedback Inhibition Regulates Amino Acid Biosynthesis
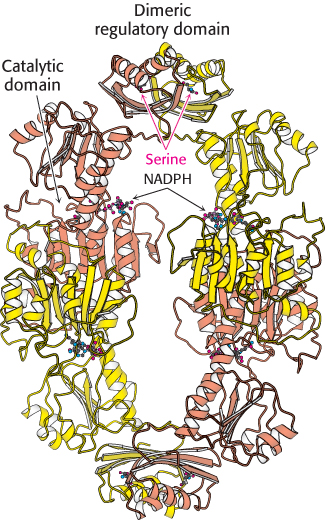
 FIGURE 24.17 Structure of 3-
FIGURE 24.17 Structure of 3-The rate of synthesis of amino acids depends mainly on the amounts of the biosynthetic enzymes and on their activities. We now consider the control of enzymatic activity. The regulation of enzyme synthesis will be discussed in Chapter 31.
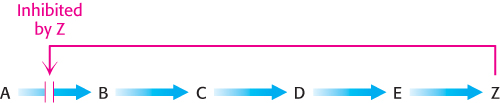
In a biosynthetic pathway, the first irreversible reaction, called the committed step, is usually an important regulatory site. The final product of the pathway (Z) often inhibits the enzyme that catalyzes the committed step (A → B).
This kind of control is essential for the conservation of building blocks and metabolic energy. Consider the biosynthesis of serine. The committed step in this pathway is the oxidation of 3-
731
Branched pathways require sophisticated regulation
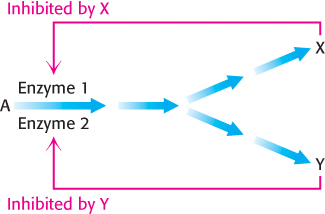
The regulation of branched pathways is more complicated because the concentration of two products must be accounted for. In fact, several intricate feedback mechanisms have been found in branched biosynthetic pathways.
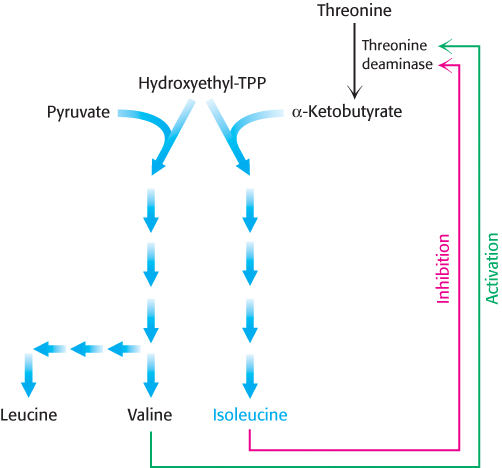
Feedback inhibition and activation. Two pathways with a common initial step may each be inhibited by its own product and activated by the product of the other pathway. Consider, for example, the biosynthesis of the branched chain amino acids valine, leucine, and isoleucine. A common intermediate, hydroxyethyl thiamine pyrophosphate (hydroxyethyl-
 The regulatory domain in threonine deaminase is very similar in structure to the regulatory domain in 3-
The regulatory domain in threonine deaminase is very similar in structure to the regulatory domain in 3-
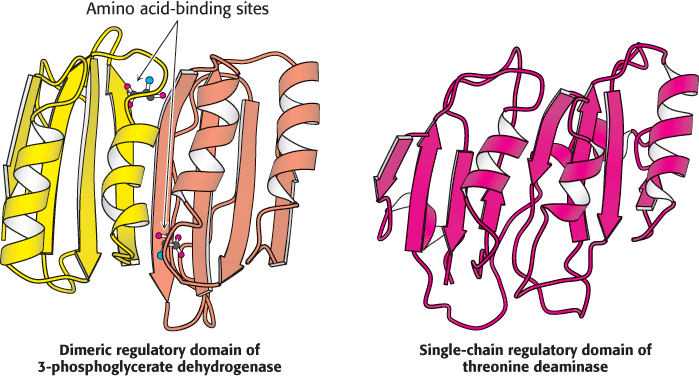
 FIGURE 24.19 A recurring regulatory domain. The regulatory domain formed by two subunits of 3-
FIGURE 24.19 A recurring regulatory domain. The regulatory domain formed by two subunits of 3-732
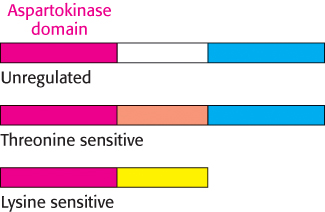
Enzyme multiplicity. The committed step can be catalyzed by two or more isozymes, enzymes with essentially identical catalytic mechanisms, but different regulatory properties (Section 10.2). For example, the phosphorylation of aspartate is the committed step in the biosynthesis of threonine, methionine, and lysine. Three distinct aspartokinases, which evolved by gene duplication, catalyze this reaction in E. coli (Figure 24.20). The catalytic domains of these enzymes show approximately 30% sequence identity. Although the mechanisms of catalysis are the same, their activities are regulated differently: one enzyme is not subject to feedback inhibition, another is inhibited by threonine, and the third is inhibited by lysine.
Cumulative feedback inhibition. A common step is partly inhibited by each of the final products, acting independently. The regulation of glutamine synthetase in E. coli is a striking example of cumulative feedback inhibition. Recall that glutamine is synthesized from glutamate, NH4+, and ATP. Glutamine synthetase consists of 12 identical 50-
The sensitivity of glutamine synthetase to allosteric regulation is altered by covalent modification
The activity of glutamine synthetase is also controlled by reversible covalent modification—the attachment of an AMP unit by a phosphodiester linkage to the hydroxyl group of a specific tyrosine residue in each subunit. This adenylylated enzyme is less active and more susceptible to cumulative feedback inhibition than is the deadenylylated form. The covalently attached AMP unit is removed from the adenylylated enzyme by phosphorolysis.
733
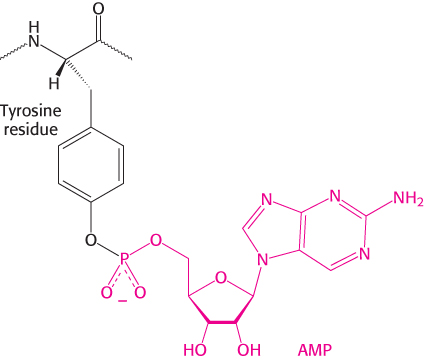
The adenylylation and phosphorolysis reactions are catalyzed by the same enzyme, adenylyl transferase. The adenylyl transferase is composed of two homologous halves, one half catalyzing the adenylylation reaction and the other half the phosphorolytic deadenylylation reaction. What determines whether an AMP unit is added or removed? The specificity of adenylyl transferase is controlled by a regulatory protein PII, a trimeric protein that can exist in two forms, unmodified (PII) or covalently bound to UMP (PII-UMP). The complex of PII and adenylyl transferase catalyzes the attachment of an AMP unit to glutamine synthetase, which reduces its activity. Conversely, the complex of PII-UMP and adenylyl transferase removes AMP from the adenylylated enzyme (Figure 24.21).
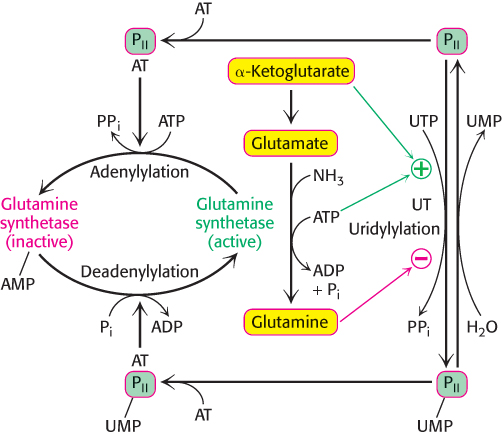
This scheme of regulation immediately raises the question, how is the modification of PII controlled? PII is converted into PII- UMP by the attachment of uridine monophosphate to a specific tyrosine residue (Figure 24.21). This reaction, which is catalyzed by uridylyl transferase, is stimulated by ATP and α-ketoglutarate, whereas it is inhibited by glutamine. In turn, the UMP units on PII are removed by hydrolysis, a reaction promoted by glutamine and inhibited by α-ketoglutarate. These opposing catalytic activities are present on a single polypeptide chain and are controlled so that the enzyme does not simultaneously catalyze uridylylation and hydrolysis. In essence, if glutamine is present, the covalent modification system favors adenylylation and inactivation of glutamine synthetase.
If glutamine is absent, as indicated by the presence of its precursors α-ketoglutarate and ATP, the control system results in the deadenylylation and activation of the synthetase. The integration of nitrogen metabolism in a cell requires that a large number of input signals be detected and processed. In addition, the regulatory protein PII also participates in regulating the transcription of genes for glutamine synthetase and other enzymes taking part in nitrogen metabolism. The evolution of covalent regulation superimposed on feedback inhibition provided many more regulatory sites and made possible a finer tuning of the flow of nitrogen in the cell. We have previously seen such a dual regulatory format in the regulation of glycogen metabolism (Section 21.5).
734