5-1 A Chemical Message
Loewi’s successful heartbeat experiment, diagrammed in Experiment 5-1, marked the beginning of research into how chemicals carry information from one neuron to another in the nervous system. Loewi was the first to isolate a chemical messenger. We now know that chemical as acetylcholine (ACh), the same transmitter that activates skeletal muscles, as described in Section 4-4. Yet here, ACh acts to inhibit heartbeat, to slow it down. It turns out that ACh activates skeletal muscles in the somatic nervous system and may either excite or inhibit various internal organs in the autonomic system. And, yes, acetylcholine is the chemical messenger that slows the heart in diving bradycardia.
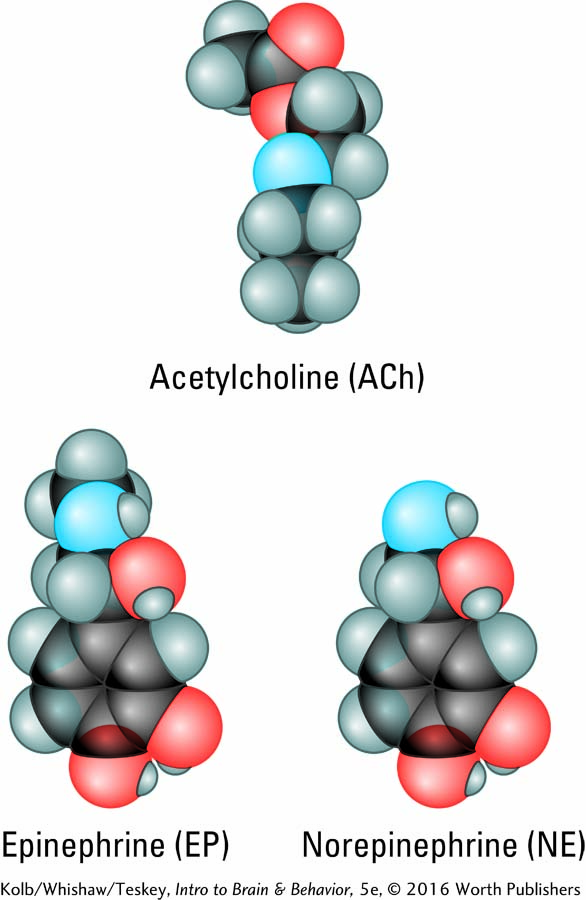
In further experiments modeled on his procedure in Experiment 5-1, Loewi stimulated another nerve to the heart, the accelerator nerve, and heart rate increased. The fluid that bathed the accelerated heart also speeded the beat of a second heart that was not electrically stimulated. Loewi identified the chemical that carries the message to speed up heart rate in frogs as epinephrine (EP; epi-, above; nephron, kidney), also known as adrenaline. Adrenaline (Latin) and epinephrine (Greek) are the same substance, produced by the adrenal glands located atop the kidneys. Adrenaline is the name more people know, in part because a drug company used it as a trade name, but epinephrine is common parlance in the science community.
EXPERIMENT
Question: How does a neuron pass on a message?
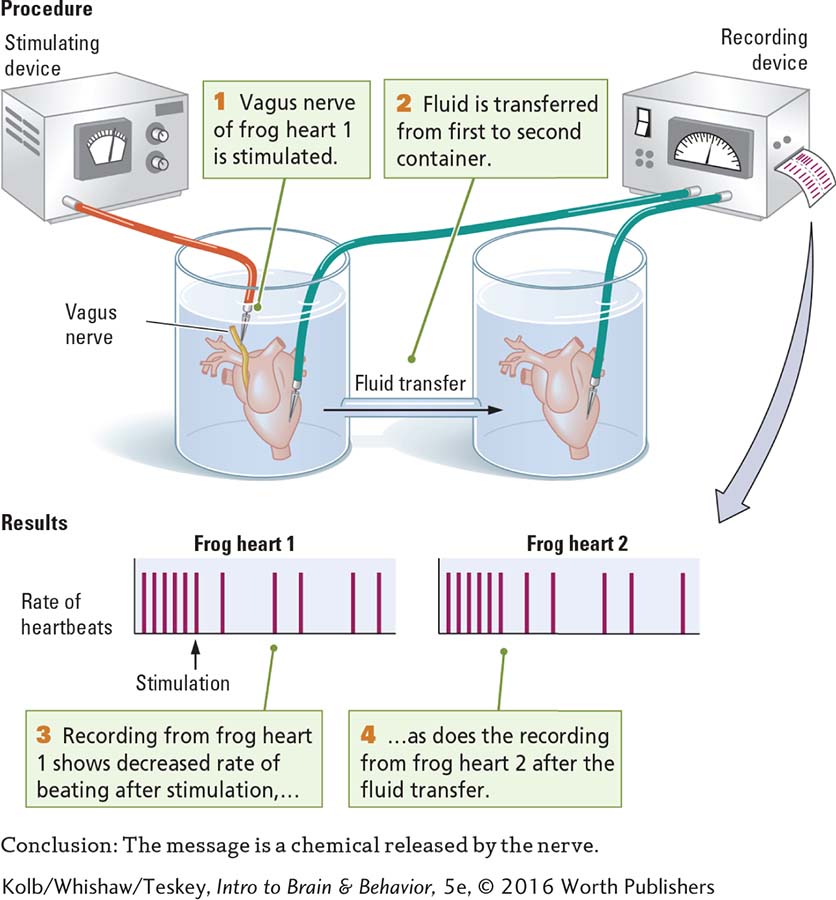
Further experimentation eventually demonstrated that in mammals, the chemical that accelerates heart rate is norepinephrine (NE; also noradrenaline), a chemical closely related to EP. The results of Loewi’s complementary experiments showed that acetylcholine from the vagus nerve inhibits heartbeat, and epinephrine from the accelerator nerve excites it.
The vagus nerve influences the heart and other internal body processes; see Figure 2-29.
Chemical messengers released by a neuron onto a target to cause an excitatory or inhibitory effect are neurotransmitters. Outside the central nervous system, many of the same chemicals, epinephrine among them, circulate in the bloodstream as hormones. Under control of the hypothalamus, the pituitary gland releases hormones into the bloodstream to excite or inhibit targets such as the organs and glands in the autonomic and enteric nervous systems. In part because hormones travel throughout the body to distant targets, their action is slower than that of CNS neurotransmitters prodded by the lightning-
Section 6-5 explains how hormones influence the brain and behavior.
Loewi’s discoveries led to the search for more neurotransmitters and their functions. The actual number of transmitters is an open question, with 100 posited as the maximum. The confirmed number is 60. Whether a chemical is accepted as a neurotransmitter depends on the extent to which it meets certain criteria. As this chapter unfolds, you will learn those criteria along with the names and functions of many neurotransmitters. You will also learn how groups of neurons form neurotransmitter systems throughout the brain to modulate, or temper, aspects of behavior. The three Clinical Focus boxes in this chapter tell the fascinating story of how one such neurotransmitter system has yielded deep insight into brain function. When depleted in a particular brain area, this neurotransmitter is associated with a specific neurological disorder. The story begins with Clinical Focus 5-2, Parkinson Disease.
5-2
Parkinson Disease
Case VI: The gentleman . . . is seventy-
In the 1817 essay from which this case study is taken, British physician James Parkinson reported similar symptoms in six patients, some of whom he had observed only in the streets near his clinic. Shaking was usually the first symptom, and it typically began in a hand. Over a span of years, the shaking spread to include the arm and then other parts of the body.
As the disease progressed, patients had a propensity to lean forward and walk on the balls of their feet. They also tended to run forward to prevent themselves from falling. In the later stages, patients had difficulty eating and swallowing. They drooled, and their bowel movements slowed. Eventually, the patients lost all muscular control and were unable to sleep because of the disruptive tremors.
More than 50 years after James Parkinson’s descriptions, French neurologist Jean-
In 1919, Constantin Tréatikoff (1974) studied the brains of nine Parkinson patients on autopsy and found that the substantia nigra, a small midbrain nucleus, had degenerated. In the brain of one patient who had parkinsonian symptoms only on one side of the body, the substantia nigra had degenerated on the side opposite that of the symptoms.
Chemical examination of the brains of Parkinson patients showed that disease symptoms appear when the level of dopamine, then a proposed neurotransmitter, was reduced to less than 10 percent of normal in the basal ganglia (Ehringer & Hornykiewicz, 1960/1974).
Confirming the role of dopamine in a neural pathway connecting the substantia nigra to the basal ganglia, Urban Ungerstedt found in 1971 that injecting a neurotoxin called 6-
hydroxydopamine into rats selectively destroyed these dopamine- containing neurons and produced symptoms of Parkinson disease.
Researchers have now linked the loss of dopamine-


Structure of Synapses
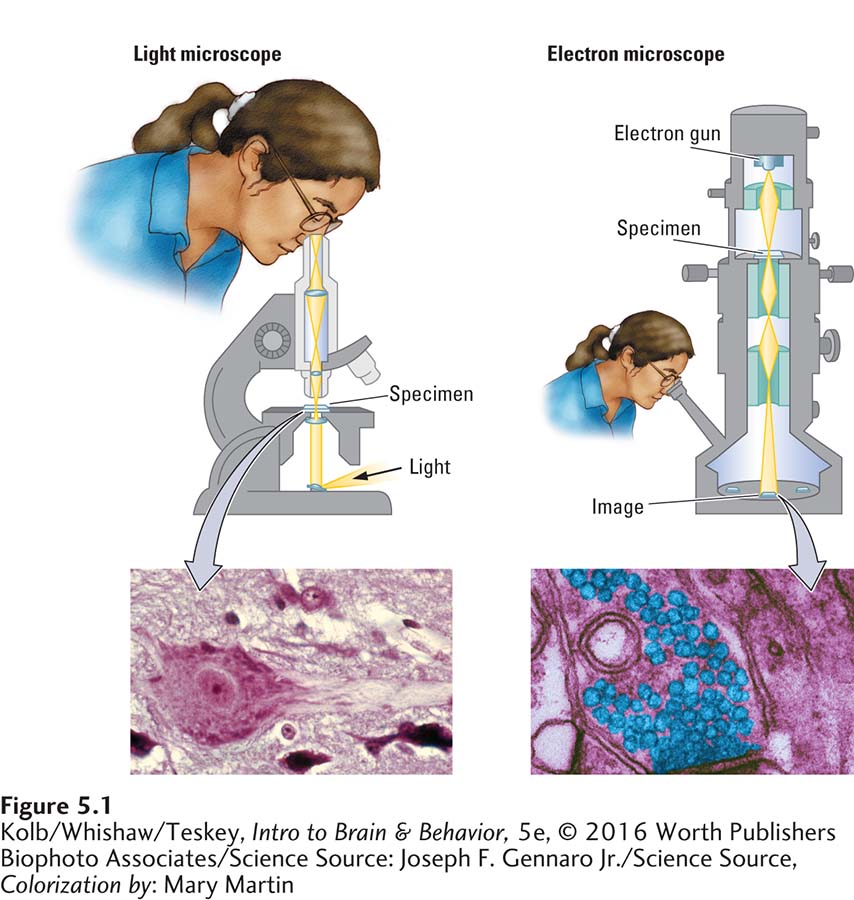
Loewi’s discovery about the chemical messengers that regulate heart rate was the first of two seminal findings that form the foundation for current understanding of how neurons communicate. The second had to wait nearly 30 years, for the invention of the electron microscope. Shown at the right in Figure 5-1, it enables scientists to see the structure of a synapse.
Joseph F. Gennaro Jr./Science Source, Colorization by: Mary Martin
Figure 4-5 describes how a digital oscilloscope measures voltage in biological tissue.
The electron microscope uses some principles of a light microscope, shown at the left in Figure 5-1, and an oscilloscope. The light microscope illuminates the tissue and magnifies the image. The electron microscope projects a beam of electrons through a very thin slice of tissue. The tissue’s varying structures scatter the electron beam onto a reflective surface, where they leave an image, or shadow, of the tissue. Electron waves are smaller than light waves and scatter much less when the beam strikes tissue, allowing for much higher resolution.
To see how much finer an electron microscope’s resolution is relative to that of a light microscope, compare the images at the bottom of Figure 5-1. An electron microscope’s resolution can be much higher than a light microscope because electron waves are smaller than light waves, so much less scatter attends the beam striking the tissue. When tissue is stained with a substance that reflects electrons, vanishingly fine structural details emerge.
Chemical Synapses
The first good electron micrographs, made in the 1950s, revealed synaptic structure for the first time. In the center of the micrograph in Figure 5-2A, the upper part of the synapse is the axon terminal, or end foot; the lower part is the receiving dendrite. The round granular substances in the terminal are the synaptic vesicles, containing neurotransmitter.
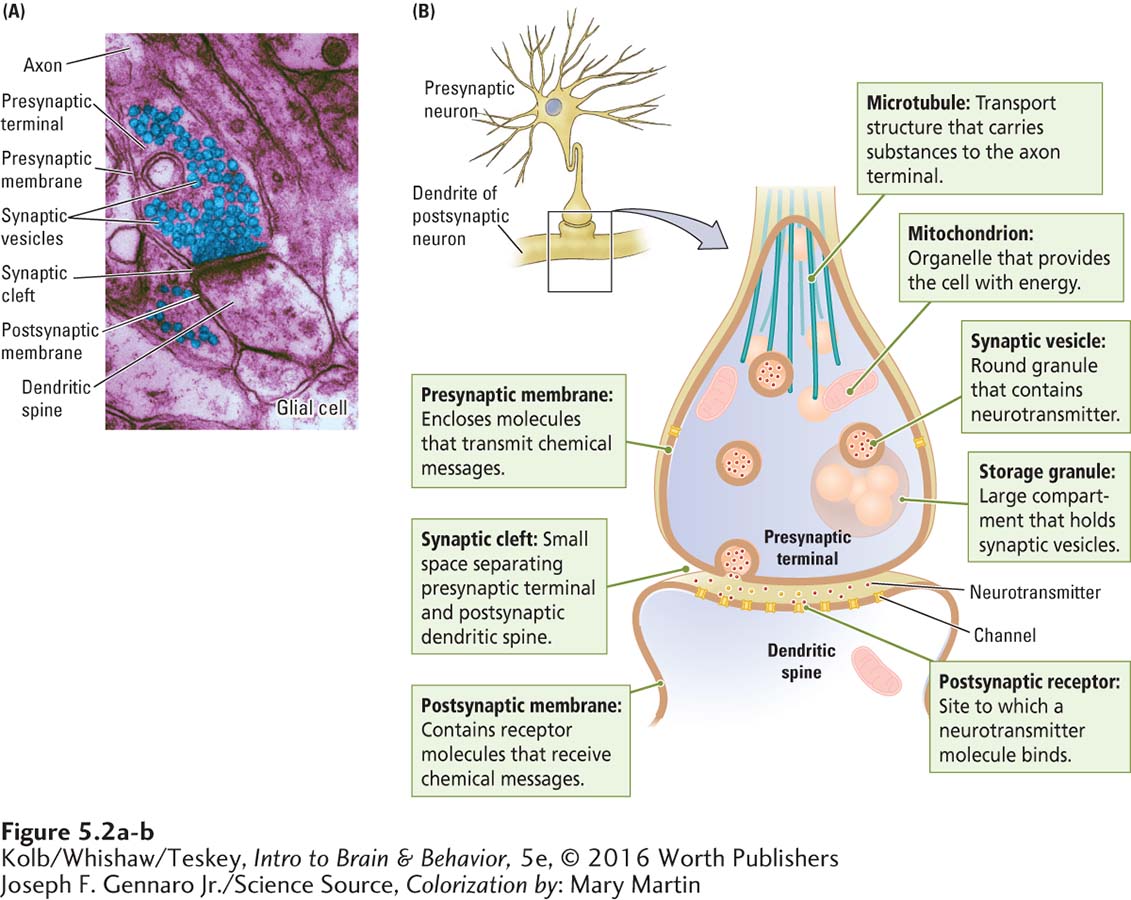
Dark patches on the axon terminal membrane are proteins that serve largely as ion channels to signal the release of the transmitters or as pumps to recapture the transmitter after its release. The dark patches on the dendrite consist mainly of receptor molecules also made up of proteins that receive chemical messages. The terminal and the dendrite are separated by a small space, the synaptic cleft. The synaptic cleft is central to synapse function, because neurotransmitter chemicals must bridge this gap to carry a message from one neuron to the next.
You can also see in the micrograph that the synapse is sandwiched by many surrounding structures, including glial cells, other axons and dendritic processes, and other synapses. The surrounding glia contribute to chemical neurotransmission in several ways—
Figure 5-2B details the process of neurotransmission at a chemical synapse, the junction where messenger molecules are released from one neuron to interact with the next neuron. Here the presynaptic membrane forms the axon terminal, the postsynaptic membrane forms the dendritic spine, and the space between the two is the synaptic cleft. Within the axon terminal are specialized structures, including mitochondria, the organelles that supply the cell’s energy needs; storage granules, large compartments that hold several synaptic vesicles; and microtubules, which transport substances, including neurotransmitter, to the terminal.
Electrical Synapses
Chemical synapses are the rule in mammalian nervous systems, but they are not the only kind of synapse. Some neurons influence each other electrically through a gap junction, or electrical synapse, where the cell membranes of two neurons come into direct contact (Figure 5-3). Ion channels in one cell membrane connect to ion channels in the other membrane, forming a pore that allows ions to pass from one neuron to the other in both directions.
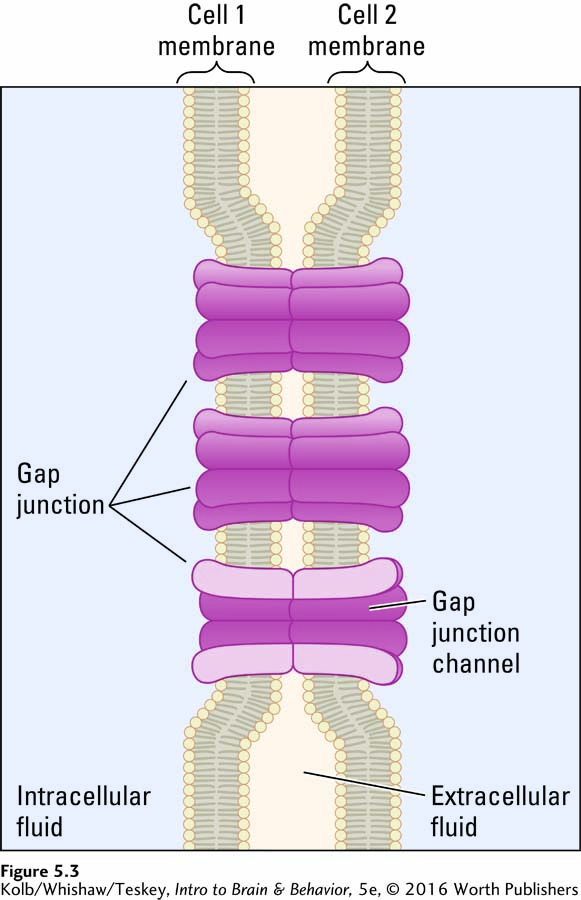
This fusion eliminates the brief delay in information flow—
Why, if chemical synapses transmit messages more slowly, do mammals rely on them more than on gap junctions? The answer is that chemical synapses flexibly control whether a message is passed from one neuron to the next; they can amplify or diminish a signal sent from one neuron to the next; and they can change with experience to alter their signals and so mediate learning.
Neurotransmission in Four Steps
The four-
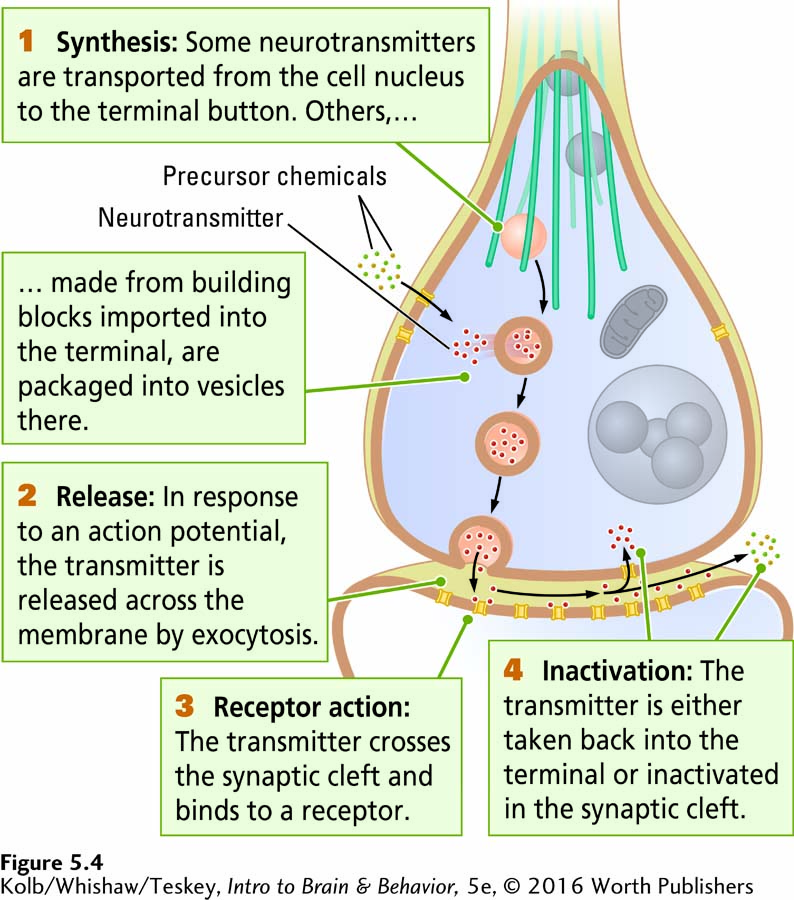
synthesized somewhere inside the neuron and stored at the axon terminal.
transported to the presynaptic membrane and released into the cleft in response to an action potential.
able to bind to and activate receptors on the postsynaptic membrane.
inactivated, so it will not continue to work indefinitely.
Step 1: Neurotransmitter Synthesis and Storage
Neurotransmitters are derived in two general ways, and these origins define two broad classes of neurotransmitters. Some are synthesized in the cell body according to instructions in the neuron’s DNA, packaged in membranes on the Golgi bodies, and transported on microtubules to the axon terminal. Cell-
Other neurotransmitters are synthesized in the axon terminal from building blocks derived from food. Transporters, protein molecules that pump substances across the cell membrane, absorb the required precursor chemicals from the blood supply. (Sometimes transporter proteins absorb the neurotransmitter ready-
For a refresher on protein export, review Figure 3-17.
Regardless of their origin, neurotransmitters in the axon terminal can usually be found in three locations, depending on their type. Some vesicles are warehoused in granules, some are attached to microfilaments (a type of microtubule; see Figure 5-2B) in the terminal, and still others are attached to the presynaptic membrane. These sites are the steps by which a transmitter is transported from a granule to the membrane, ready to be released into the synaptic cleft.
Step 2: Neurotransmitter Release
Synaptic vesicles loaded with neurotransmitters must dock near release sites on the presynaptic membrane. Then the vesicles are primed to prepare them to fuse rapidly in response to calcium (Ca2+) influx. When an action potential reaches the presynaptic membrane, voltage changes on the membrane set the release process in motion. Calcium cations play a critical role. The presynaptic membrane is rich in voltage-
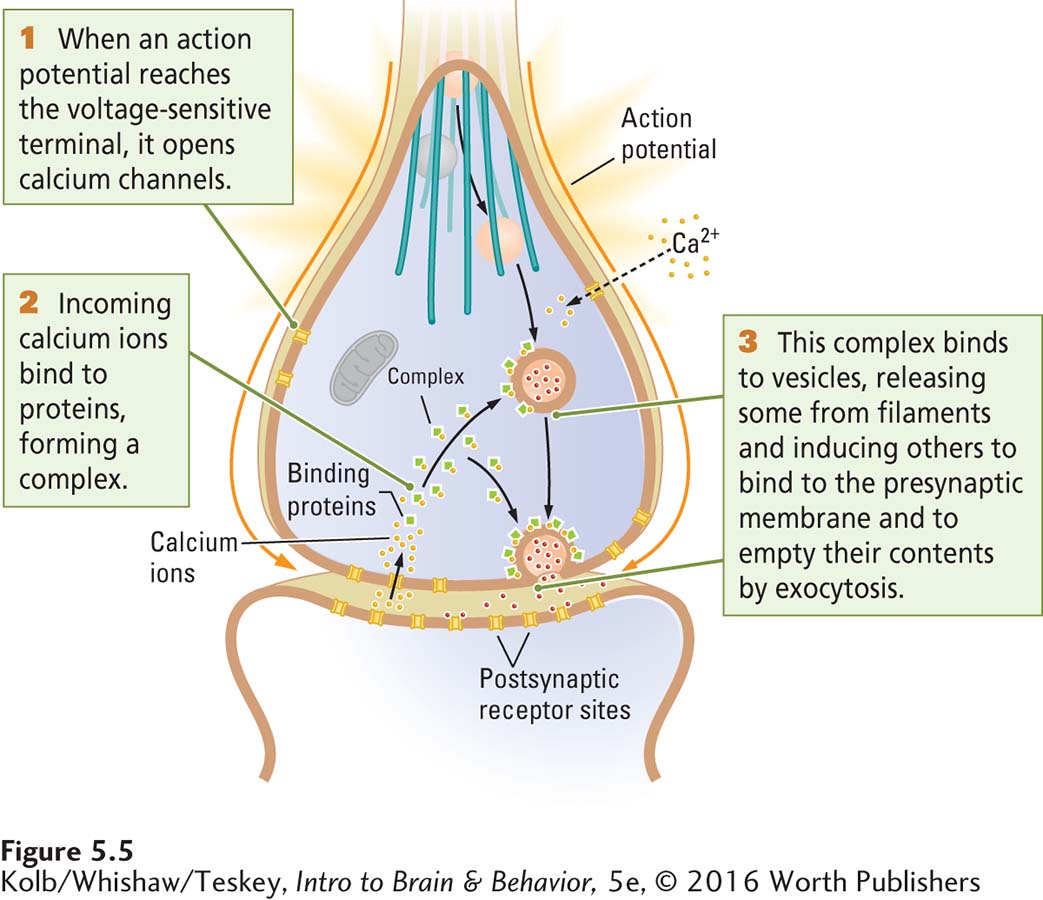
Primed vesicles fuse with the presynaptic membrane quickly in response to the calcium influx and empty their contents into the synaptic cleft by exocytosis. The vesicles from storage granules and on filaments then move up to replace the vesicles that just emptied their contents.
Step 3: Receptor-
After its release from vesicles on the presynaptic membrane, the neurotransmitter diffuses across the synaptic cleft and binds to specialized protein molecules embedded in the postsynaptic membrane. These transmitter-
depolarize the postsynaptic membrane and so have an excitatory action on the postsynaptic neuron.
hyperpolarize the postsynaptic membrane and so have an inhibitory action on the postsynaptic neuron.
initiate other chemical reactions that modulate either effect, inhibitory or excitatory, or that influence other functions of the receiving neuron.
In addition to interacting with the postsynaptic membrane’s receptors, a neurotransmitter may interact with receptors on the presynaptic membrane: it may influence the cell that just released it. That is, a neurotransmitter may activate presynaptic receptors called autoreceptors (self-
How much neurotransmitter is needed to send a message? Bernard Katz was awarded a Nobel Prize in 1970 for providing an answer. Recording electrical activity from the postsynaptic membranes of muscles, he detected small, spontaneous depolarizations now called miniature postsynaptic potentials. The potentials varied in size, but each size appeared to be a multiple of the smallest potential.
Katz concluded that the smallest postsynaptic potential is produced by the release of the contents of just one synaptic vesicle. This amount of neurotransmitter is called a quantum. To produce a postsynaptic potential large enough to initiate a postsynaptic action potential requires the simultaneous release of many quanta from the presynaptic cell.
The results of subsequent experiments show that the number of quanta released from the presynaptic membrane in response to a single action potential depends on two factors: (1) the amount of Ca2+ that enters the axon terminal in response to the action potential and (2) the number of vesicles docked at the membrane, waiting to be released. Both factors are relevant to synaptic activity during learning, which we consider in Section 5-4.
Step 4: Neurotransmitter Deactivation
Chemical transmission would not be an effective messenger system if a neurotransmitter lingered within the synaptic cleft, continuing to occupy and stimulate receptors. If this happened, the postsynaptic cell could not respond to other messages sent by the presynaptic neuron. Thus, after a neurotransmitter has done its work, it is quickly removed from receptor sites and from the synaptic cleft. Deactivation is accomplished in at least four ways:
Diffusion. Some of the neurotransmitter simply diffuses away from the synaptic cleft and is no longer available to bind to receptors.
Degradation. Enzymes in the synaptic cleft break down the transmitter.
Reuptake. Membrane transporters specific to that transmitter may bring it back into the presynaptic axon terminal for reuse. The by-
products of degradation by enzymes also may be taken back into the terminal to be used again in the cell. Astrocyte uptake. Some neurotransmitters are taken up by neighboring astrocytes. Potentially, the astrocytes can also store transmitters for re-
export to the axon terminal.
Table 3-1 describes astrocytes and other types of glial cells and their functions.
Highlighting the flexibility of synaptic function, an axon terminal has chemical mechanisms that enable it to respond to the frequency of its own use. If the terminal is very active, the amount of neurotransmitter made and stored there increases. If the terminal is not often used, however, enzymes within the terminal buttons may break down excess transmitter. The by-
Varieties of Synapses
So far we have considered a generic chemical synapse, with features possessed by most synapses. Synapses vary widely in the nervous system. Each type is specialized in location, structure, function, and target. Figure 5-6 illustrates this diversity on a single hypothetical neuron.
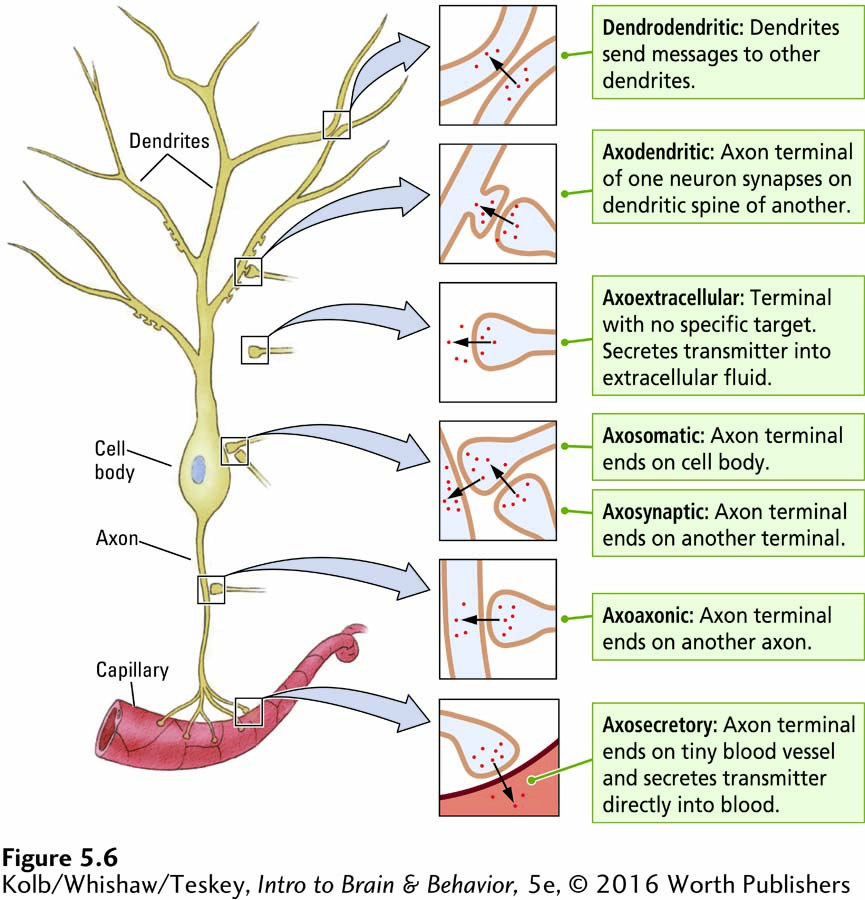
Figure 4-26 shows both microscopic and schematic views of an axomuscular synapse
You have already encountered two kinds of synapses. One is the axomuscular synapse, in which an axon synapses with a muscle end plate, releasing acetylcholine. The other synapse familiar to you is the axodendritic synapse, detailed in Figure 5-2B, in which the axon terminal of a neuron synapses with a dendrite or dendritic spine of another neuron.
Figure 5-6 diagrams axon terminals at the axodendritic synapse as well as the axosomatic synapse, at a cell body; the axoaxonic synapse, on another axon; and the axosynaptic synapse, on another presynaptic terminal—
This wide variety of connections makes the synapse a versatile chemical delivery system. Synapses can deliver transmitters to highly specific sites or diffuse locales. Through connections to the dendrites, cell body, or axon of a neuron, transmitters can control the neuron’s actions in different ways.
Through axosynaptic connections, they can also exert exquisite control over another neuron’s input to a cell. By excreting transmitters into extracellular fluid or into the blood, axoextracellular and axosecretory synapses can modulate the function of large areas of tissue or even the entire body. Many transmitters secreted by neurons act as hormones circulating in your blood, with widespread influences on your body.
More about hormones in Section 6-5.
Gap junctions, shown in Figure 5-3, further increase the signaling diversity between neurons. Such interneuronal communication may occur via dendrodendritic and axoaxonic gap junctions. Gap junctions also allow neighboring neurons to synchronize their signals through somatosomatic (cell body to cell body) connections, and they allow glial cells, especially astrocytes, to pass nutrient chemicals to neurons and to receive waste products from them.
Excitatory and Inhibitory Messages
Each neuron receives thousands of excitatory and inhibitory signals every second. The nervous system works by juxtaposing these signals.
A neurotransmitter can influence a neuron’s functioning through a remarkable variety of mechanisms. In its direct actions in influencing a neuron’s electrical activity, however, a neurotransmitter acting through its receptors has only one of two effects. It influences transmembrane ion flow either to increase or to decrease the likelihood that the cell with which it comes in contact will produce an action potential. Thus, despite the wide variety of synapses, they all convey messages of only these two types, excitatory or inhibitory. To be precise, neurotransmitters themselves do not determine excitation or inhibition. The receptor makes the call.
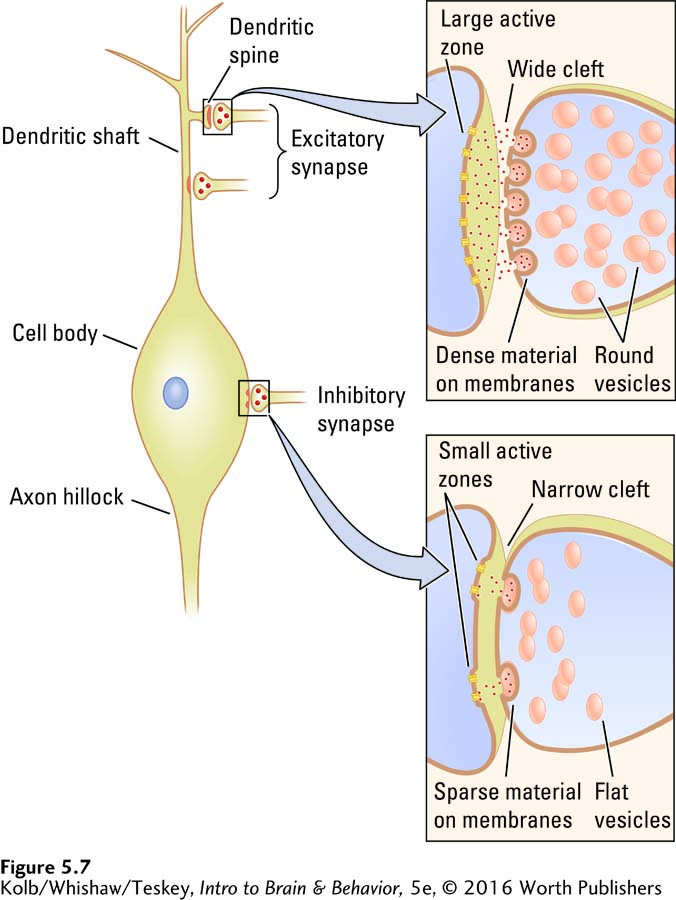
Excitatory and inhibitory synapses differ in their appearance and are generally located on different parts of the neuron. As shown in Figure 5-7, excitatory synapses are typically on the shafts or spines of dendrites, whereas inhibitory synapses are typically on the cell body. Excitatory synapses have round synaptic vesicles; the vesicles in inhibitory synapses are flattened. The material on the presynaptic and postsynaptic membranes is denser in an excitatory synapse than it is in an inhibitory synapse, and the excitatory synaptic cleft is wider. Finally, the active zone on an excitatory synapse is larger than that on an inhibitory synapse.
Behaviors are lost when a disorder prevents excitatory instructions and released when a disorder prevents inhibitory instructions.
The differing locations of excitatory and inhibitory synapses divide a neuron into two zones: an excitatory dendritic tree and an inhibitory cell body. Think of excitatory and inhibitory messages as interacting from these two different perspectives. Viewed from an inhibitory perspective, you can picture excitation coming in over the dendrites and spreading past the axon hillock to trigger an action potential at the initial segment. If the message is to be stopped, it is best stopped by inhibiting the cell body close to the initial segment. In this model of excitatory–
Another way to conceptualize excitatory–
Evolution of Complex Neurotransmission Systems
Considering all the biochemical steps required for getting a message across a synapse and the variety of synapses, you might well wonder why—
To make the origin of chemical secretions for neuronal communication easier to imagine, think about the feeding behaviors of simple single-
The exocytosis mechanism for digestion in a single-
5-1 REVIEW
A Chemical Message
Before you continue, check your understanding.
Question 1
In mammals, the principal form of communication between neurons occurs via ______, even though this structure is slower and more complex than the fused _________.
Question 2
The principal benefit of chemical synapses over electrical synapses is that they can change with ________ to alter their signals and so mediate ______.
Question 3
The nervous system has evolved a variety of synapses:
________ between axon terminals and dendrites,
________ between axon terminals and cell bodies,
________ between axon terminals and muscles,
________ between axon terminals and other axons,
________ between axon terminals and other synapses.
A(n) _______ synapse releases chemical transmitters into extracellular fluid, a(n) ________ synapse releases transmitter into the bloodstream as hormones, and still another, the ______ synapse, connects dendrites to other dendrites.
Question 4
Excitatory synapses are usually located on a(n) _____ , whereas inhibitory synapses are usually located on a(n) ______.
Question 5
Describe the four steps in chemical neurotransmission.
Answers appear in the Self Test section of the book.