15.6 G Protein–Coupled Receptors That Trigger Elevations in Cytosolic and Mitochondrial Calcium
Calcium ions play an essential role in regulating cellular responses to many signals, and many GPCRs and other types of receptors exert their effects on cells by influencing the cytosolic concentration of Ca2+. As we saw in Chapter 11, the level of Ca2+ in the cytosol is tightly maintained at a submicromolar level (0.1 µM = 100 nM) by the continuous action of ATP-
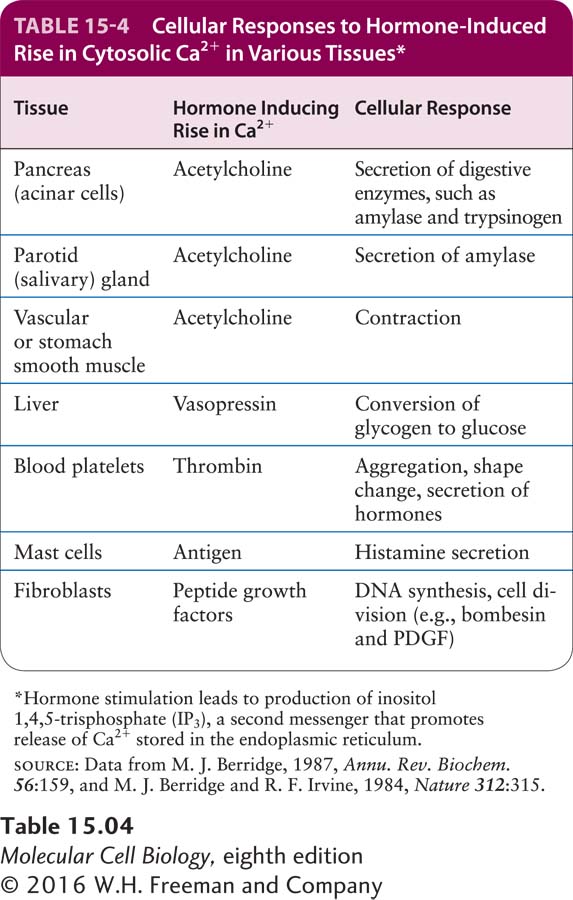
A small rise in cytosolic Ca2+ induces a variety of cellular responses, including hormone secretion by endocrine cells, secretion of digestive enzymes by pancreatic acinar cells, and contraction of muscle (Table 15-4). For example, acetylcholine stimulation of GPCRs in secretory cells of the pancreas and of the parotid (salivary) glands induces a rise in cytosolic Ca2+ that triggers the fusion of secretory vesicles with the plasma membrane and release of their protein contents into the extracellular space. Thrombin, an enzyme in the blood-
We learned in Chapter 12 that increases in the concentration of free Ca2+ in the mitochondrial matrix accelerate pyruvate oxidation and ATP production. Thus, in muscle, increases in the concentration of Ca2+ are used both to induce contractions and to coordinately increase mitochondrial ATP synthesis to provide the energy to fuel those contractions.
In this section, we first discuss experimental tools scientists use to measure the concentration of free Ca2+ ions—
Page 709
Some PLCs are activated by GPCRs, as we describe here; others, covered in the following chapter, are activated by other types of receptors. Phospholipases C also produce second messengers that are important for remodeling the actin cytoskeleton (see Chapter 17) and for the binding of proteins important for endocytosis and vesicle fusion (see Chapter 14). Later in this section, we will see how second messengers such as Ca2+ are used to help cells integrate their responses to more than one extracellular signal. In the final part of the section, we see how one PLC pathway leads to the synthesis of a gas, nitric oxide (NO), which diffuses out of the cell into adjacent cells, where it can induce activation of a kinase that, in turn, alters several cellular activities.