CONCEPT31.1 Respiratory Gas Exchange Depends on Diffusion and Bulk Flow
The respiratory gases that animals must exchange with their environment are oxygen (O2) and carbon dioxide (CO2). Cells require O2 from the environment so they can produce ATP by cellular respiration (see Concept 6.2). Specifically, O2 is needed in the mitochondria of each cell because electrons removed from food molecules must have a final resting place after they pass through the mitochondrial electron transport chain, releasing the energy that the mitochondria use to make ATP. Their final resting place is in combination with O2 to make water (see Figure 6.9). This water simply mingles with the rest of the water in an animal’s body.
Cellular respiration also produces CO2 because CO2 is one of the products of the citric acid cycle (see Figure 6.8). Unlike the H2O produced by cellular respiration, CO2 cannot be allowed simply to accumulate in an animal’s body water. CO2 is often called a “gaseous acid” because it tends to lower the pH of the body fluids if it accumulates in them. It can also have other disadvantageous effects. For these reasons, as an animal takes in O2 from its environment and delivers it to cells for use in cellular respiration, the animal must rid itself of the CO2 made by cellular respiration by releasing the CO2 into its environment.
LINK
You can review aerobic cellular respiration and the production of ATP in Concept 6.2
Think about the path an O2 molecule must travel from the atmosphere to a mitochondrion inside a muscle cell in your calf. First the O2 molecule must travel deep into your lungs. Then it must cross two simple epithelia to enter the blood: first the epithelium forming your lung wall and then the epithelium forming the wall of one of your blood capillaries. The molecule must then travel in the blood from your thorax to your leg to reach a blood capillary next to the muscle cell. Then, to enter the cell, the molecule must cross the wall of the blood capillary (an epithelium) and pass through the cell membrane of the muscle cell. Finally, the molecule must travel through the cell cytoplasm to reach and enter a mitochondrion. CO2 must make essentially the same journey in reverse.
Notice that some of the steps in an O2 molecule’s trip from atmosphere to mitochondrion are very short (FIGURE 31.1). The distance across each epithelium is about 1 micrometer (μm) or less. The distance across a cell membrane is roughly 0.01 μm. The distance from the cell membrane of a muscle cell to the deepest mitochondrion inside the cell is likely to be only 5–50 μm.
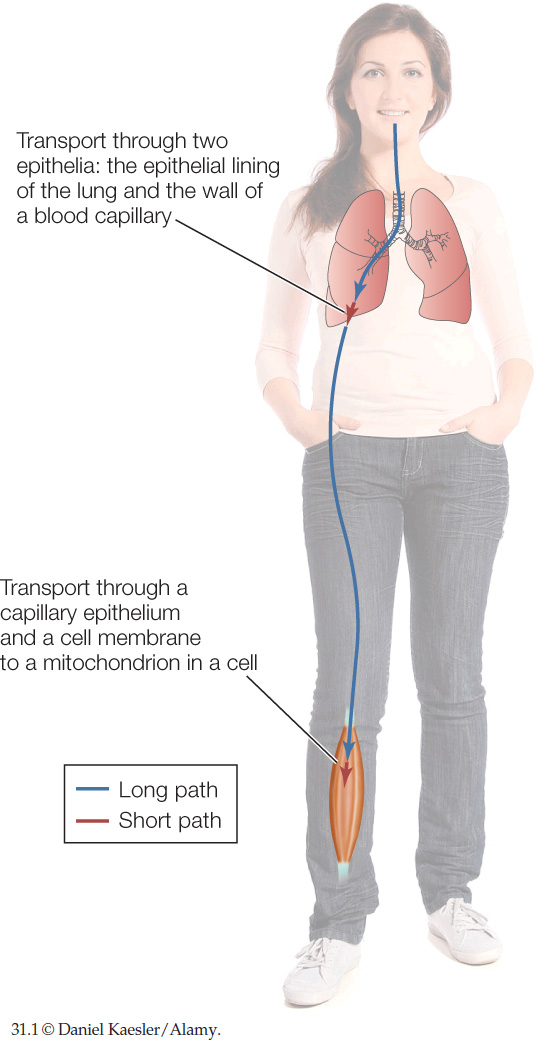
By contrast, some of the other steps in an O2 molecule’s trip are extraordinarily long. To pass deep into your lungs from your nostrils, an O2 molecule needs to travel about 500,000 μm (0.5 meter). To travel in your blood from your lungs to a muscle cell in your calf, the molecule needs to travel about 1,000,000 μm!
Distance is therefore one of the factors we need to take into account as we consider the travels of O2 and CO2 molecules between the atmosphere and an animal’s cells.
Also note this important point: active transport mechanisms for O2 do not exist (see Concept 5.3). This means that energy supplied by ATP cannot be used to drive O2 through an epithelium or cell membrane. Instead, O2 always crosses epithelia and cell membranes by diffusion. Much the same can be said for CO2, although active transport of CO2 is known in certain cases in the animal kingdom.
The diffusion of gases depends on their partial pressures
Atoms and molecules undergo ceaseless random motions at atomic–molecular scales of space. Diffusion results from these random motions (see Concept 5.2). If a certain type of molecule is more concentrated in one region of a solution than another, it is a simple statistical fact that random motions will carry more molecules away from the concentrated region than they will carry into it. In this way, the random motions cause a net transport of molecules from the concentrated region to neighboring dilute regions. This is the principle by which O2 and CO2 diffuse in a solution.
LINK
See Concept 5.2 for further information about the diffusion of molecules in solution
645
Complexities arise where liquids and gases are in contact. When O2 is present in a gas mixture, it is simply one of the types of gas molecules. For example, in air 21 percent of the molecules are O2. When O2 is present in a liquid, it is in solution. You have seen that when you dissolve sugar in water, the sugar molecules become invisible. O2 does exactly the same thing. When it dissolves in water, the individual O2 molecules fit in between water molecules and become invisible (bubbles, regardless of how tiny, are not in solution).
What happens when O2 diffuses from a gas into an aqueous (watery) solution—so that the O2 molecules switch from being in a gaseous to a dissolved state—or vice versa? How can we predict whether this diffusion will be from the gas to the liquid or in the opposite direction? Chemists have discovered that if you measure a property called partial pressure, you can solve this problem. O2 always diffuses from where its partial pressure is high to where its partial pressure is low. Partial pressure is easily defined in a gas phase (not so easily in a liquid phase): it’s simply the portion of the total pressure exerted by any particular gas, such as O2, in a mixture of gases.
To clarify the relationship between concentration and partial pressure, suppose we have a gas phase in contact with a liquid phase, as in FIGURE 31.2. Within a gas phase, the concentration and partial pressure of O2 are proportional to each other. O2 always diffuses from where its concentration is high to where its concentration is low, for the reasons already discussed. Because concentration and partial pressure are proportional, O2 also diffuses from where its partial pressure is high to where its partial pressure is low. Within a liquid phase, the same statements are true. Concentration and partial pressure are proportional, and O2 always diffuses from places where both are high to places where both are low.
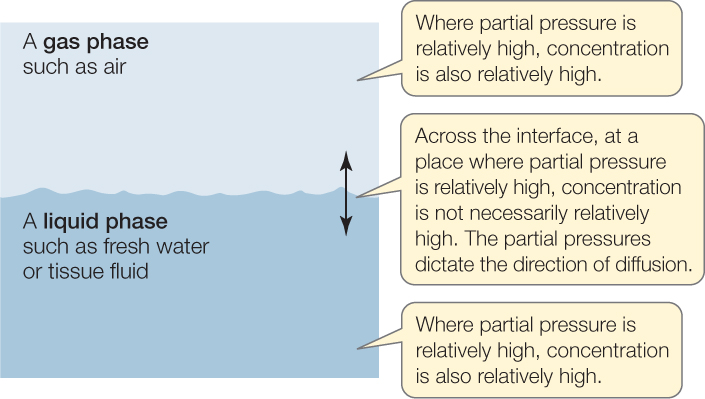
However, when O2 diffuses between a gas phase and a liquid phase, its direction of diffusion cannot be predicted from its concentrations in the two phases. When diffusing from one phase to the other, O2 moves from where its partial pressure is high to where it is low, even though this direction may be opposite to what concentrations would suggest (the partial pressures have the advantage that they take into account the effects of the solubility of O2 in water, which the concentrations do not take properly into account). The same principles apply to all other gases, such as CO2 and N2.
The law of diffusion for gases—often called Fick’s law—is expressed by the equation
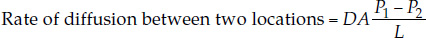
where
- D, the diffusion coefficient, depends on the diffusion medium (air or water), the temperature, and the particular gas that’s diffusing
- A is the cross-sectional area across which the gas is diffusing
- P1 and P2 are the partial pressures of the gas at the two locations
- L is the distance (diffusion path length) between the two locations
This equation applies to the diffusion of all gases, and it applies within a gas phase, within a liquid phase, and between gas and liquid phases. Partial pressures are expressed in units of pressure. Thus, the standard unit for partial pressure is the pascal (Pa). Alternative units in common use are atmospheres (atm) and millimeters of mercury (mm Hg), the latter referring to the height of a mercury column the pressure can support (1 atm = 760 mm Hg = 101.3 kilopascals).
Diffusion can be very effective but only over short distances
Let’s ask the simple question, Can diffusion move lots of O2 (or CO2) from one place to another in a short period of time? The answer is both yes and no. This distinction is one of the most important concepts to understand about diffusion. As we’ll see, the answer depends on the distance involved.
Notice that the distance between two locations, L, appears in the denominator of the diffusion equation. The rate of diffusion between two locations increases as the distance between the locations decreases.
Suppose O2 on the outside of a cell is diffusing across the cell membrane into the cell. The distance, L, for this diffusion is very short—about 0.01 μm. Thus this diffusion will be very fast. In fact, if you were to arrange to have a high O2 concentration outside a cell and a low concentration inside, diffusion would move enough O2 into the cell in just 0.1 microsecond to halve the concentration difference between outside and inside! This explains why all cells depend simply on diffusion for O2 to pass into them through their cell membranes.
By contrast, suppose O2 present in body fluids near your lungs is diffusing through your body fluids to a muscle cell in your calf. For this diffusion, L is much longer—about 1 meter. This diffusion therefore will be slow—far slower, in fact, than you might ever have imagined. If you had a high O2 concentration near your lungs and a low concentration in your calf muscle, diffusion would require more than 30 years to halve the concentration difference! Diffusion would obviously be totally useless for getting O2 to your calf muscle.
646
We see, then, that diffusion can be very effective for moving O2 over short distances but not over long distances. The founder of modern respiratory physiology, August Krogh, calculated an important rule of thumb for understanding diffusion. He analyzed a system in which O2 had to diffuse through tissue to reach mitochondria that were carrying out cellular respiration at an average rate for animals, and he asked how thick the tissue could be. His answer was about 0.5 millimeter. Today, physiologists still use Krogh’s rule of thumb and view 0.5 mm as the greatest distance over which O2 can diffuse fast enough through tissue or tissue fluids to meet metabolic needs.
Gas transport in animals often occurs by alternating diffusion and bulk flow
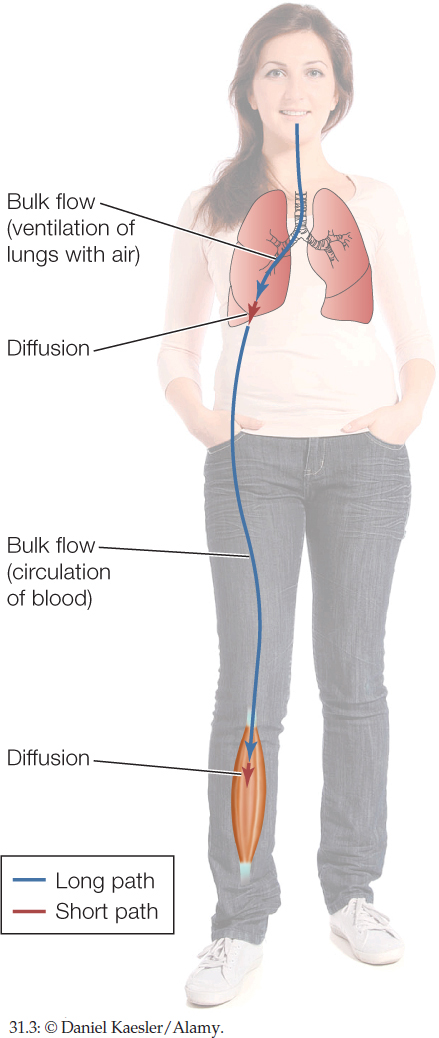
Returning to Figure 31.1, we can see that diffusion is suitable for moving O2 through the short steps in the path from the atmosphere to a muscle cell. It is not suitable, however, for the long steps.
This limitation explains why animals have often evolved processes of bulk flow to transport O2 over long distances. Bulk flow refers to a flow of matter from one place to another. Winds and water currents are forms of bulk flow in the physical world. When you breathe, you create a bulk flow of air into and out of your lungs. The beating of your heart causes a bulk flow of blood through your blood vessels. Bulk flow can carry O2 over long distances at high rates.
LINK
The systems that circulate blood throughout the bodies of animals are described in Concept 32.1 and Concept 32.2
Diffusion and bulk flow alternate in humans and other mammals (FIGURE 31.3). O2 is carried by bulk flow over the long distance from the atmosphere to the depths of the lungs. Then it diffuses across the thin epithelia in the lungs to pass into the blood. O2 is then again carried by bulk flow, in the circulating blood, to reach the legs. Finally, O2 diffuses out of blood in the legs to cross a blood-capillary epithelium, cross a cell membrane, and finally reach a mitochondrion.
Breathing is the transport of O2 and CO2 between the outside environment and gas exchange membranes
The gas exchange membranes of an animal are thin layers of tissue—usually comprising only one or two simple epithelia—where the respiratory gases move between the animal’s environmental medium—air or water—and its internal tissues. Our own gas exchange membranes, for example, are the membranes deep in our lungs where O2 is removed from the air and enters our blood and where CO2 leaves our blood and enters the air. All animals have gas exchange membranes of some type. O2 moves into the internal tissues from the layer of environmental medium immediately next to the gas exchange membranes, and CO2 moves from the internal tissues into that layer.
How does O2 from the outside world get to the layer of environmental medium next to the gas exchange membranes? And how does CO2 move from that layer to the outside world? These questions are the subject of the study of breathing.
Breathing—also called external expiration—is the process by which O2 and CO2 are transported between the layer of environmental medium next to the gas exchange membranes and the outside world. Ventilation refers to bulk flow of air or water between the gas exchange membranes and the outside world. Breathing often occurs by ventilation. This is true in humans and most other vertebrates, for example. However, breathing can occur entirely by diffusion if the distances to be covered are short. Many tiny insects, such as gnats, are believed to breathe by diffusion.
Physiologists categorize breathing organs into two fundamental types: lungs and gills (FIGURE 31.4). Lungs are invaginated (folded inward) into the body, and their passages contain the environmental medium. Gills are evaginated (folded outward) from the body and are surrounded by the environmental medium. In general, air-breathing animals have lungs and water-breathing animals have gills, although exceptions occur.
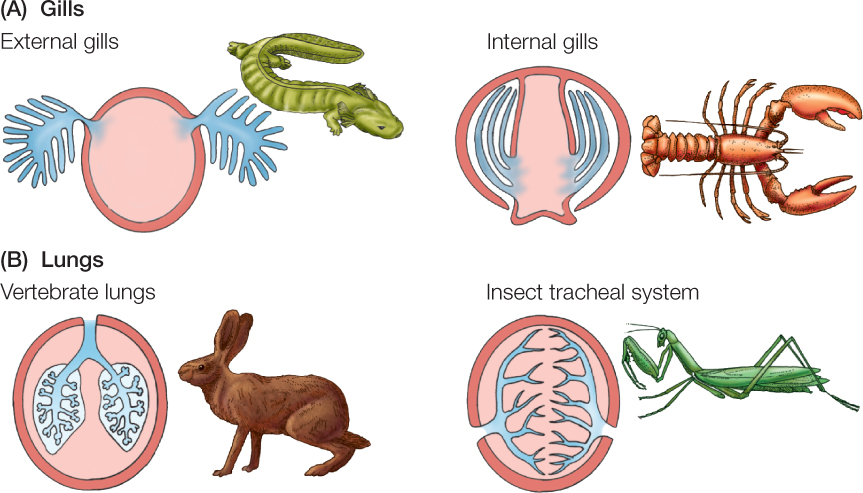
When lungs are ventilated, air usually moves into them along lung passages during inhalation and then moves out through the same passages during exhalation. This ventilation is described as tidal because air flow occurs first in one direction and then in the opposite direction in the same airways—in and out like the ocean tides. Humans exemplify tidal ventilation.
647
In animals with gills, breathing usually involves pumping water in a one-way stream over the gill surfaces. This water movement is the ventilation of the gills, and the ventilation is described as unidirectional.
Air and water are very different respiratory environments
Diffusion takes place much faster through air than water (a fact reflected by the value of D in the diffusion equation). If all factors that affect diffusion are kept constant except that diffusion may be through air or water, the rate of diffusion of O2 will be about 200,000 times faster through air!
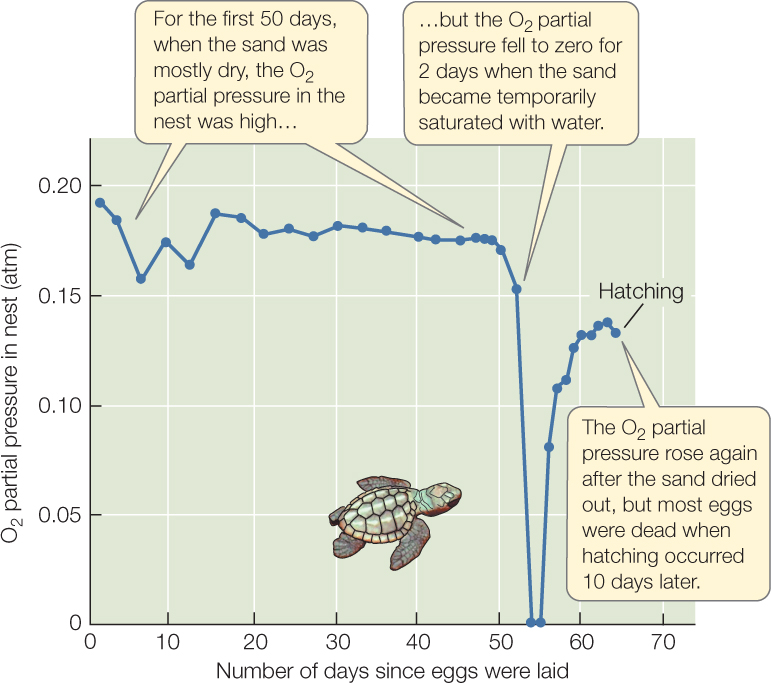
Sea turtle nests that become flooded provide a striking illustration of the difference in diffusion rates through air and water (FIGURE 31.5). Loggerhead sea turtles lay their eggs about 40 centimeters deep on ocean beaches and cover them with sand. Nathan Miller outfitted nests with tiny probes that continually monitored the partial pressure of O2 in the nests. He found that the eggs received plenty of O2 by diffusion through the sand from the atmosphere above, provided the spaces between sand grains were full of air (Krogh’s rule does not apply in air). However, if the sand became flooded with water for a long period—so the spaces between sand grains were water-filled—the O2 partial pressure in the nest could fall to zero, causing the death of many eggs.
In addition to its slow rate of diffusion through water, O2 also is not very soluble in water. Suppose we have some fresh water in an aquarium and we bubble it continuously with air so that the water and air are at equilibrium with each other (in this state they have the same O2 partial pressure). At 12°C the air will contain about 200 milliliters (ml) of O2 in each liter. The water, however, will contain only about 7.7 ml of O2 in each liter! The solubility of O2 in water is so low that the amount present per liter is only about 4 percent as high as in air—even when the water is steadily bubbled with air.
The solubility of O2 in water decreases as water temperature rises. Thus the concentration of O2 in water tends to decrease as water warms. Fresh water at 24°C, for example, dissolves 6.2 ml of O2 in each liter, substantially less than the 7.7 ml per liter dissolved at 12°C. Most aquatic animals are poikilothermic (see Concept 29.3). Thus as water warms, their body temperatures and rates of O2 consumption increase (see Figure 29.6). At the same time, the amount of dissolved O2 available from each liter of water decreases. Water-breathing animals can find themselves caught in a respiratory trap, in which their O2 needs increase as O2 availability decreases. This can weaken them—interfering with growth or reproduction—and even kill them if the mismatch between O2 needs and O2 availability becomes too great.
648
Respiratory gas exchange depends on diffusion and bulk flow
The need for O2 and the availability of O2 can clash in fish as water temperatures rise. Water contains much less O2 than an equal volume of air, and it requires more effort to be pumped over respiratory gas exchange surfaces. Another factor that affects respiration in water-breathing animals is temperature, which affects both their O2 needs and the solubility of O2 in water. Answer the following questions by constructing a graph from the data in the table. Use water temperature as your x axis.
- How does water temperature influence the O2 available to a water-breathing animal?
- Why does water temperature affect the O2 demands of a fish?
- From the data, what is the evidence that O2 availability is a limiting factor for metabolism in warm water?
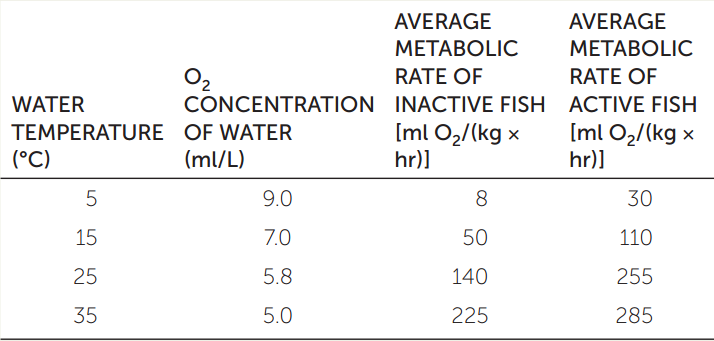
- From the data, what general argument would you make about the effect of environmental warming on the viability of aquatic species?
All the animals alive today with the highest metabolic rates are air-breathers. Water is a challenging place to live in terms of getting enough O2, if an animal is a water-breather.
CHECKpointCONCEPT31.1
- Where in the human body do inhaled O2 molecules first cross a cell membrane?
- Review the equation for Fick’s law in Concept 31.1. What happens to the rate of diffusion as A increases? As L increases?
- Many ocean fish depend on the phenomenon of upwelling, where cold, nutrient-rich water rises up from the depths to mix with warm, nutrient-poor water near the surface. Which has the greater potential to store O2, warm water or cold water? And how might the concentration of O2 influence a fish’s ability to metabolize nutrients available in its environment?
Now that we have examined the basics of respiratory gas exchange, let’s look at the diversity of structures and processes by which animals obtain O2 and get rid of CO2.