Predation and herbivory favor the evolution of defenses
Given the large effects that predators can have on their prey and herbivores can have on producers, it is not surprising that many species have evolved strategies to defend themselves. In this section, we will review the types of defenses that have evolved and how they function. We will also look at how some predators and herbivores have evolved counterdefenses.
Defenses Against Predators
To understand the defenses prey use against predators, we first need to understand predator hunting strategies. Predator hunting strategies can be categorized as either active hunting or ambush hunting, also known as sit-and-wait hunting. A predator that uses active hunting spends most of its time moving around looking for potential prey. For example, American robins actively hunt when they move around a lawn searching for earthworms. In contrast, a predator that uses ambush hunting lies in wait for a prey to pass by. Chameleons can sit very still as they wait for an insect to pass. When the insect is close enough, the chameleon shoots out its long tongue and the sticky, prehensile tip grabs the unsuspecting prey.
Regardless of hunting mode, we can think of hunting by predators as a series of events: detecting the prey, pursuing the prey, catching the prey, handling the prey, and consuming the prey. As we will see, prey have evolved defenses to thwart the predator at different points in this series of events.
Behavioral Defenses
Some of the most common behavioral defenses against predators include alarm calling, spatial avoidance, and reduced activity. Alarm calling is used by many species of birds and mammals to warn their relatives that predators are approaching. Prey that use spatial avoidance move away from the predator. If the predator moves toward them, they continue to move away from the danger. Prey that follow a strategy of reduced activity are less likely to come into contact with a predator. When the prey detect the presence of a predator, they reduce their movements, which minimizes the chance of being detected. In a study of six species of tadpoles, a researcher placed animals in tubs of water with one of two treatments. The first contained a caged predator that could not kill the tadpoles but could emit chemical cues to scare the tadpoles. The second was a control treatment that consisted of an empty cage. Once the experiment was set up, the researcher watched the tadpoles to determine their activity level, which was defined as the percent of the time that the animals spent moving. As shown in Figure 14.15, each species exhibited a different level of activity when predators were absent and all species reduced their level of activity in the presence of the predator.
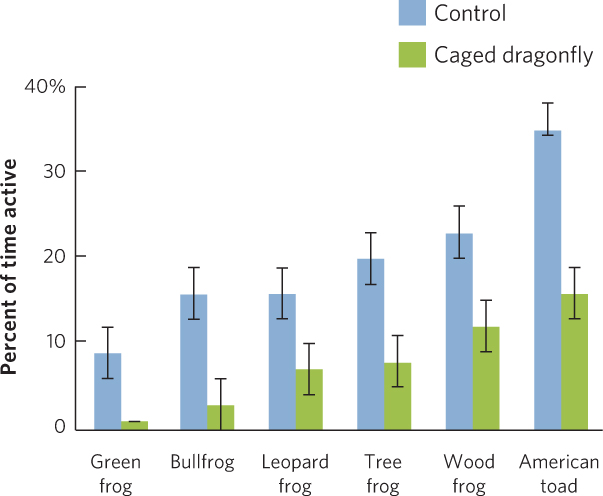
332
Crypsis
Crypsis Camouflage that either allows an individual to match its environment or breaks up the outline of an individual to blend in better with the background environment.
Another way to reduce the probability of being detected by a predator is through camouflage that either matches the environment or breaks up the outline of an individual to blend in better with the background environment, a phenomenon known as crypsis. Various animals resemble sticks, leaves, flower parts, or even bird droppings. These organisms are not so much concealed but mistaken for inedible objects and passed over by predators. Common species that use crypsis include stick insects, katydids, and horned lizards (Figure 14.16). Some species have a fixed color pattern that aids in crypsis while other species, such as the octopus, are able to rapidly change color in ways that make themselves match their background.

Structural Defenses
Although some prey use behavior and crypsis to avoid being detected, other species employ mechanical defenses that reduce the predator’s ability to capture, attack, or handle the prey. One of the best-known examples of a mechanical defense is the barbed quills of the porcupine; more than 30,000 quills cover the porcupine’s body and can penetrate the flesh of an attacking predator. In other species, the structural defenses are phenotypically plastic and therefore only induced when the prey detects a predator in the environment. For example, water fleas—tiny freshwater crustaceans—that detect the chemical cues of predators early in their life can develop spines along different parts of their body to deter predators from consuming them. Other mechanical defenses involve changes in the overall shape of the body. For example, the crucian carp (Carassius carassius), a species of fish that lives in Europe and Asia, develops a deep, hump-shaped body over a period of many weeks when the carp smells a predatory fish in the water. As you can see in Figure 14.17, carp with a hump-shaped body have greater muscle mass and can accelerate more quickly away from a predator.
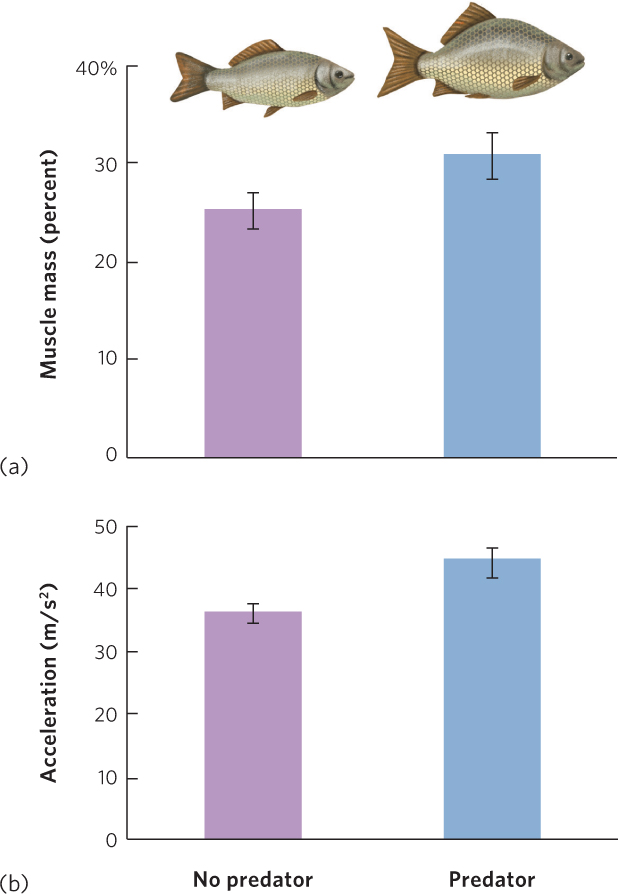
333
Chemical Defenses
Prey can also use chemical defenses to deter a predator. Skunks, well-known for using this strategy, spray potential threats with foul-smelling chemicals from posterior glands. Many insects also use chemical defenses. For example, when monarch butterfly caterpillars feed on milkweed, they store some of the milkweed toxins in their body, which make the butterfly very distasteful to predatory birds. Bombardier beetles take a different approach. Their abdomens contain two glands, each of which makes a distinct chemical. When agitated, the beetle mixes these two chemicals, causing a reaction that makes the liquid approach 100° C. They shoot the boiling hot liquid out of the abdomen, causing pain or death to small predators, as illustrated in Figure 14.18.

Warning coloration A strategy in which distastefulness evolves in association with very conspicuous colors and patterns. Also known as Aposematism.
While chemical defenses are often effective in deterring predation, these defenses are even more effective if the prey can communicate their distastefulness to the predator before an attack occurs. In many species, distastefulness has evolved in association with very conspicuous colors and patterns, a strategy known as warning coloration, or aposematism. Predators quickly learn to avoid markings such as the black and orange stripes of monarch butterflies; the insect tastes so bitter that a single experience is well-remembered. Conspicuous combinations of black, red, and yellow adorn such diverse animals as bombardier beetles, yellow-jacket wasps, and coral snakes. These color combinations consistently advertise noxiousness; some predators have evolved innate aversions to them and do not need to go through the process of learning to avoid a particular prey.
Mimicry of Chemical Defenses
Batesian mimicry When palatable species evolve warning coloration that resembles unpalatable species.
When predators avoid aposematic species, any individuals of palatable species that resemble the distasteful, aposematic species would also be favored by selection. Over generations, the palatable species can evolve to more closely resemble the aposematic species. When palatable species evolve warning coloration that resembles unpalatable species, we call it Batesian mimicry, named for Henry Bates, the nineteenth-century English naturalist who first described it. In his journeys to the Amazon region of South America, Bates found numerous cases of palatable insects that did not retain the cryptic patterns of their close relatives but instead had evolved to resemble brightly colored, unpalatable species (Figure 14.19).

334
Experimental studies have demonstrated that mimicry does confer an advantage to the mimics. For example, toads that consumed live bees and received a sting on the tongue subsequently avoided palatable drone flies, which mimic the appearance of bees. In contrast, when naive toads consumed dead bees with stingers removed, they consumed the bees and the drone flies. This result indicated that toads learned to associate the conspicuous and distinctive color patterns of live bees with an unpleasant experience.
Müllerian mimicry When several unpalatable species evolve a similar pattern of warning coloration.
Another type of mimicry, called Müllerian mimicry, occurs when several unpalatable species evolve a similar pattern of warning coloration. Müllerian mimicry is named after its discoverer, the nineteenth-century German zoologist Fritz Müller. When multiple species of prey have conspicuous color patterns and all are unpalatable, a predator that learns to avoid one prey species will later avoid all prey species with a similar appearance. For example, most of the bumblebees and wasps that co-occur in mountain meadows share a pattern of black and yellow stripes and they all have the ability to sting a predator. Similarly, in Peru several species of poison dart frogs, all in the genus Ranitomeya, closely resemble each other. In four regions of that country researchers have found three species that vary in coloration according to location, including one species (R. variabilis) that looks very different across two locations. A fourth species, R. imitator, is also unpalatable and has populations in each of these four locations that closely resemble the other species that is present (Figure 14.20).

Costs of Defenses Against Predators
Many types of defenses against predators can be costly, as we discussed in our coverage of predator- and herbivore-induced defenses in Chapter 4. For example, because behavioral defenses frequently result in reduced feeding activity or increased crowding in locations away from predators, behavioral defenses often come at the cost of reduced growth and development. Similarly, most mechanical defenses are energetically expensive to produce, such as the 30,000 quills of a porcupine. In some cases, the costs of defense are so high that they can come at the cost of growth and reproduction. When this happens, the presence of predators can cause smaller population sizes even when they do not consume the prey.
Less is known about the costs of chemical defenses in prey, but recent studies suggest that these defenses are also energetically costly to produce. In ladybugs, which are technically known as ladybird beetles, many species are red with black spots. These warning colors communicate to predators that the ladybugs taste bad because of chemicals in their bodies known as alkaloids. However, there is a large amount of variation in the concentration of alkaloids that each ladybug can produce. In 2012, researchers reported that only beetles that consumed large amounts of food had sufficient energy to produce high concentrations of carotenoid chemicals in their bodies, which give the ladybugs a more intense red color, as shown in Figure 14.21a. Moreover, as illustrated in Figure 14.21b, beetles that produced more carotenoids also produced higher concentrations of alkaloids. As a result, ladybugs with the highest energy diet can better advertise their level of toxicity to predators and thereby reduce their chances of being attacked.
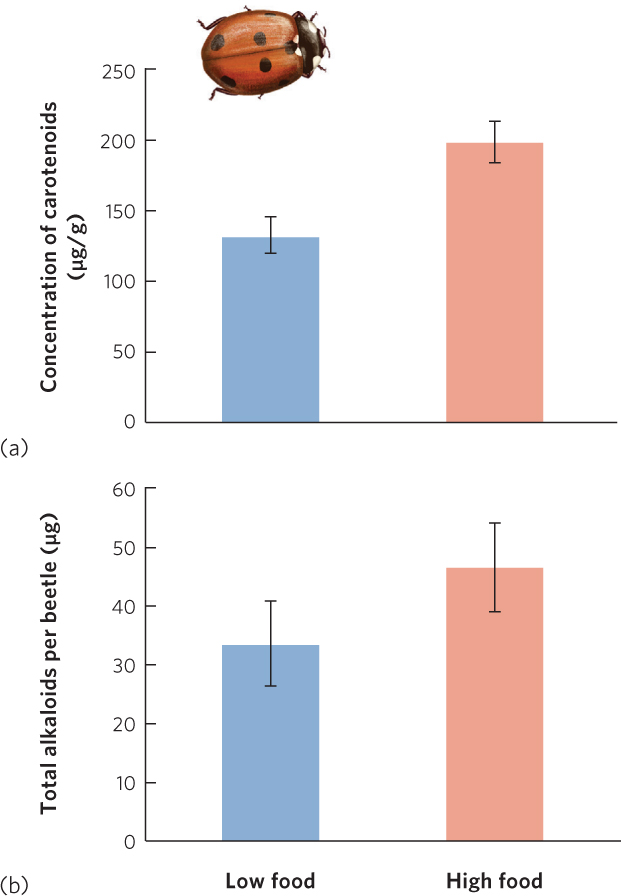
335
336
Counter Adaptations of Predators
Coevolution When two or more species affect each other’s evolution.
If predation can select for prey to evolve a wide range of defenses, then prey defenses should favor the selection for counter-adaptation in predators. In this way, predators and prey experience an evolutionary arms race between prey defenses and predator offenses. When two or more species affect each other’s evolution, we call it coevolution. In the case of the porcupine, for example, the spines deter most predators. However, bobcats (Lynx rufus) and wolverines (Gulo gulo) have an effective solution. When these predators find a porcupine, they flip the porcupine on its back and attack the belly, which is not defended by spines. Other common predator adaptations include high-speed locomotion to catch their prey and camouflage that allows them to ambush their prey.
Some predators can also evolve to handle the toxic chemicals produced by prey. For example, the cane toad (Bufo marinus) is a species that was introduced into Australia in 1935. Like other species of toads, the cane toad contains toxins in its skin that can cause predators to become sick or die. As a result, predators in the native range of cane toads do not attack cane toads even though these same predators regularly consume other species of amphibians. When the toads were introduced to Australia, predators of amphibians, such as black snakes (Pseudechis porphyriacus), had no evolutionary experience with cane toads. When they ate the toads, most snakes had no resistance to the toxins and they died. In 2006, however, researchers reported that some populations of the black snakes were consuming cane toads, which suggests that while most black snakes that consumed cane toads died, some snakes must have been resistant to the toad’s toxins and survived. Over time, the selection for snake resistance to the toad toxin must have resulted in the evolution of resistant populations. To test this hypothesis, the researchers fed samples of toad skin to snakes from different populations around Australia. They then examined how much the toad skin reduced the swimming speed of each snake, which allowed the researchers to determine the susceptibility of each snake to the toxin. As you can see in Figure 14.22, the researchers found that snake populations that coexisted with cane toads for the longest amount of time evolved the lowest susceptibility to the cane toad toxin. This evolution would have occurred in less than 70 years, which is a remarkably short time.
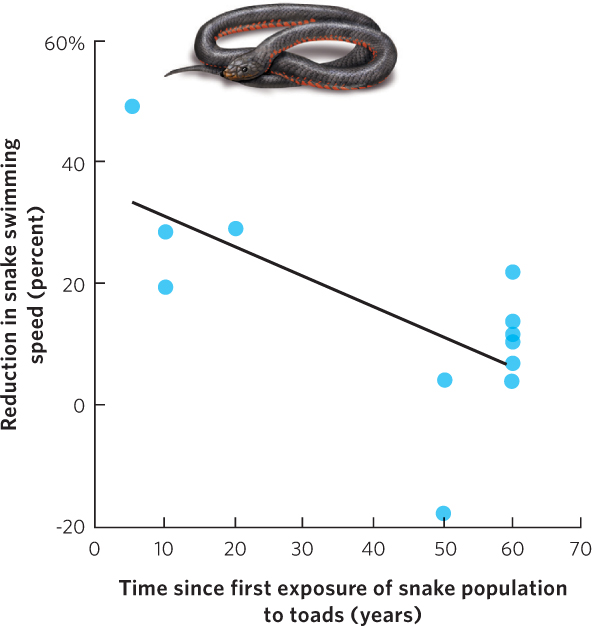
Defenses Against Herbivores
The selective pressure from predators has caused the evolution of prey defenses. Similarly, continued selective pressure from herbivores has caused the evolution of defenses against herbivory. In some cases, these defenses are induced by an herbivore attack and are therefore phenotypically plastic. In other cases, plant defenses are fixed and therefore are expressed whether or not an herbivore has attacked the plant. In both cases, some herbivore species have evolved counter-adaptations. In fact, some species of herbivores are so specialized in countering the defenses of a particular plant species that they do not consume any other plants.
Structural Defenses
When it comes to structural defenses, plants have evolved a variety of traits to deter herbivores from consuming their leaves, stems, flowers, or fruit. Some plants, such as cacti, roses, and blackberries, have sharp spines and prickles that inflict pain on the mouths of herbivores. Other plants grow a wooly layer of hair over their leaf surfaces to make it difficult for herbivorous insects to penetrate them.
Chemical Defenses
A wide variety of chemical defenses have evolved in plants. Plant chemicals include sticky resins and latex compounds that are hard to consume. Some plants also produce alkaloids—including caffeine, nicotine, and morphine—that have a wide range of toxic effects on herbivores. Other chemicals in plants such as tannins are difficult for herbivores to digest. It is generally thought that the chemicals produced by plants are the by-products of a plant’s physiology.
337
ANALYZING ECOLOGY
Understanding Statistical Significance
In examining adaptations of prey, counter-adaptations of predators, or any other ecological measurements, we often look at experiments in which researchers find differences among experimental manipulations. Until now, we have not explored how ecologists assess when such differences are meaningful versus when the differences are due to chance.
For any group of measurements, such as the concentration of toxins in ladybugs fed high and low amounts of food, there will be variation among the individuals of each group. If we were to sample ladybugs at random from the high- and low-food treatment, we would find that the mean toxin concentration is higher in the high-food treatment. However, the measurements taken on some of the individuals in the high-food treatment could overlap with the measurements taken on some of the individuals in the low-food treatment. When the means are similar and the distribution of the data from two groups are almost entirely overlapping, we would have to conclude that the two groups are nearly identical in whatever we are measuring, as shown in the figure below.
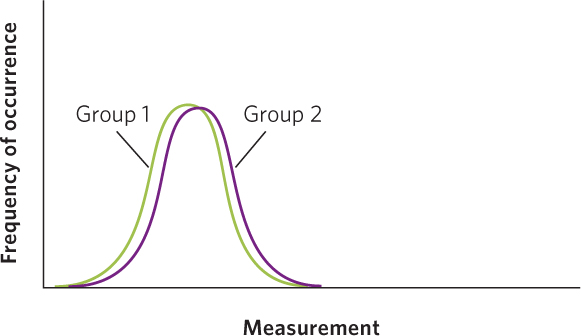
In contrast, when the means are very far apart and the distribution of the data from two groups exhibit no overlap, as in the case below, we would feel confident that we have two completely different groups of individuals.
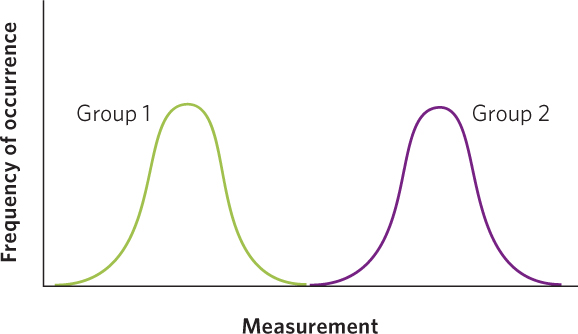
Although these two extreme alternatives demonstrate when two groups have completely overlapping or completely nonoverlapping distributions, we need to know how much overlap between the two groups is acceptable in order to conclude that the groups are different from each other.
Scientists agree that two distributions can be considered “significantly different” if we can sample the two distributions many times and find that the means of those distributions overlap less than 5 percent of the time. This somewhat arbitrary, but widely accepted, cutoff value is known as alpha (α). Thus, we say that our cutoff for statistical significance is α < 0.05. Determining that something has statistical significance is not the same as stating that a difference between two means is large, substantial, or important. In other words, the everyday use of “significant” is not synonymous with the scientific use of “significantly different.”
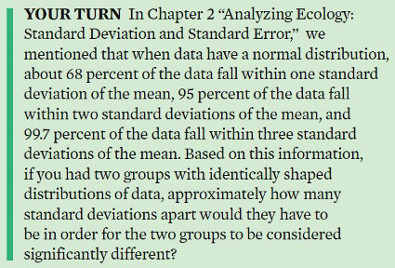
When we repeatedly sample the two distributions, we can conclude that they are significantly different ifthe two means do not overlap 95 percent of the time. This would happen when the two distributions are approximately two standard deviations apart fromeach other.
For many of these chemical defenses, one or more species of herbivore has evolved tolerance. For example, a shrub in Polynesia called the Tahitian noni (Morinda citrifolia) produces toxic chemicals with such a foul smell that the plant has the nickname “vomit fruit” (Figure 14.23). Most species of fruit flies avoid this plant because if they were to land on it, they would die. However, one species of fruit fly (Drosophila sechellia) can tolerate the defensive chemicals. It lays its eggs on the vomit flower and they have a distinct fitness advantage because they experience no competition from other species of fruit flies.

338
A similar situation exists for the monarch butterfly. As mentioned in our discussion of prey defenses, the monarch caterpillar specializes in feeding on milkweed plants, which produce toxic chemicals. The monarch caterpillar readily feeds on milkweed plants because it has evolved to resist the effects of a group of chemicals known as cardiac glycosides, which can stop the heart of many other herbivores. It also sequesters some of the chemicals to serve as a defense against its own predators. In 2012, researchers made a striking discovery about the evolution of insects’ ability to consume the plants containing cardiac glycosides. They found that a diversity of insects from different orders—including flies, beetles, true bugs, and butterflies—independently evolved the same changes in a gene that offers resistance to the effects of the toxin. In short, the different groups of insects exhibited convergent evolution.
Tolerance
Some plants that have not evolved extensive defenses against herbivores take an alternative strategy of tolerating herbivory. Plants taking this strategy are able to grow new tissues rapidly to replace those that are consumed. For example, herbivores often consume the top meristem of a plant, which is the region of the plant where most growth occurs. When this meristem is removed, the meristems of lower stems begin to experience increased rates of growth, which still allows the plant to experience relatively high fitness despite being partially consumed by an herbivore.
Costs of Herbivore Defenses
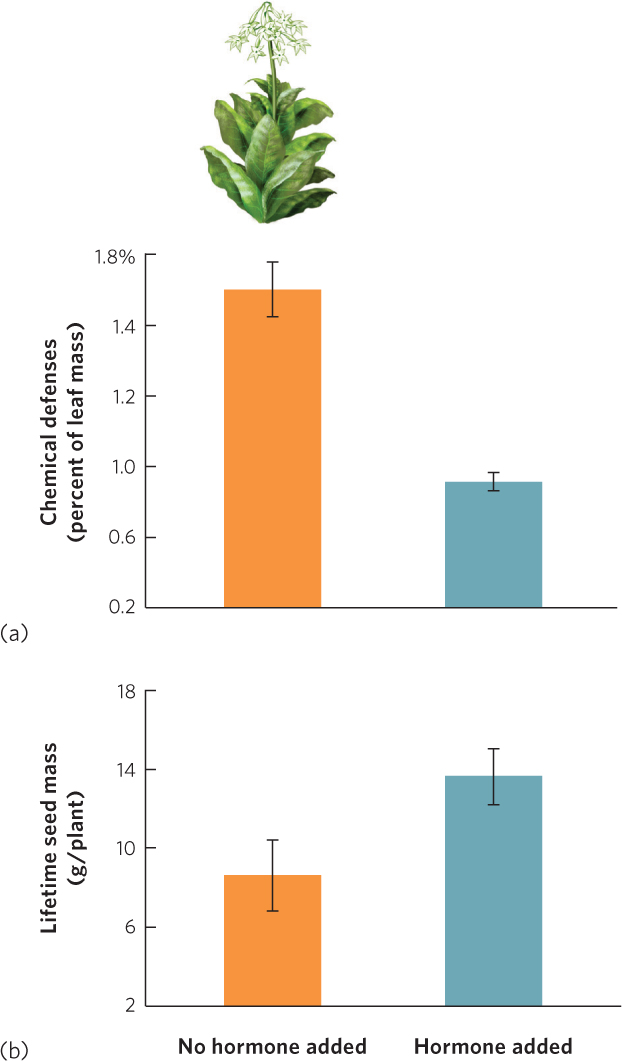
For decades, researchers have investigated whether plant defenses come at the cost of reduced fitness. When defense traits are phenotypically plastic, researchers can compare the fitness of individuals with induced defenses against the fitness of noninduced individuals. For example, tobacco plants (Nicotiana sylvestris) respond to herbivory by producing chemical defenses including nicotine. Researchers damaged one group of tobacco plants to induce an increase in chemical defenses. As a control, they also damaged a second group of plants and then treated the damaged areas with a plant hormone that prevented the chemical defenses from responding. When they later counted the number of seeds produced by the two groups, they found that the group with increased chemical defenses produced fewer seeds, as you can see in Figure 14.24.
A second approach to quantifying the costs of defenses against herbivores is to make the genes responsible for defenses nonfunctional. For instance, researchers have examined the growth of different genotypes of mouse-ear cress, a tiny plant that is native to Europe and Asia. In 2011, they reported that individuals with intact defense genes commonly grew more slowly than individuals with nonfunctional defense genes. This confirmed that plants pay a cost for fixed defenses against herbivores.
339
ECOLOGY TODAY CONNECTING THE CONCEPTS
THE TROUBLE WITH CATS AND RABBITS

Islands throughout the world often contain a variety of endemic species that have evolved together for millions of years. As we have seen in this chapter, new species that are introduced to islands can have devastating effects on the native plants and animals. Because the native species do not share an evolutionary history with the introduced species, they have not evolved defenses against introduced predators and herbivores. These introductions happen commonly on islands. One typical response is an attempt to remove the intruders and thereby reverse the harmful effects, although the actual outcomes of these efforts can lead to unintended consequences.
An example of this occurred on Macquarie Island, a small island located halfway between Australia and Antarctica. Island plants and animals existed together for eons in a tundra biome that contained a large number of seabirds and land birds, as well as a diversity of plants that included tall grasses. Humans began to visit the island in the 1800s and they brought several species of animals with them.
In the early 1800s, the island was used by seal hunters as a place to rest and to resupply their ships. These visitors introduced house cats (Felis catus), which soon became feral. While there are no data on the impact of the feral cats, it is generally assumed that they acted as a mesopredator and preyed upon the abundant island birds. In 1878, the seal hunters introduced European rabbits (Oryctolagus cuniculus) to the island to serve as a food source whenever the sailors returned to the island. Despite the fact that the cats fed on the rabbits, over time the rabbit population grew to very large numbers. Data collected from the 1950s through the 1970s suggest that the rabbit population experienced large fluctuations approximately every 10 years, similar to those seen in hares and other animals that live in high northern latitudes. Because the plants on the island had no evolutionary history with the rabbits, periods of high rabbit numbers caused devastating effects on the abundance of palatable plant species; when the rabbit population experienced large declines, the vegetation rebounded.
340
Because periods of high rabbit populations caused such adverse effects on the island’s vegetation, scientists introduced the European rabbit flea (Spilopsyllus cuniculi) in 1968. The fleas can carry a Myxoma virus that causes the disease myxomatosis, which is fatal to rabbits. The scientists quickly learned that the virus did not persist well on the island, so beginning in 1978 they reintroduced it every year. The virus had its desired effect and caused the rabbit populations to plunge from a high of 130,000 individuals in 1978 to fewer than 20,000 by the 1980s. As the rabbit population declined, the vegetation of the island began to recover. However, the changes to the island community did not end there.
With the rapid decline of the rabbits, the cats of the island exhibited prey-switching behavior—described in our discussion of predator functional responses—and added more birds to their diet. They consumed an estimated 60,000 seabirds per year and caused the extinction of two species of endemic land birds. To help save the island’s undefended birds, government officials decided to eradicate all of the cats from the island.
Starting in 1985, scientists removed 100 to 200 cats from the island each year. An analysis of the cat stomachs in 1997 indicated that while the cat population was killing birds, the cat population was still consuming approximately 4,000 rabbits per year, so the cats—in combination with the Myxoma virus— were probably still regulating the rabbit population.
When the last cat was removed in 2000, the island community responded rapidly. For example, five species of seabirds started breeding on the island, some of which had not bred there for more than 80 years. The rabbits also responded. From 1985 to 2000, the rabbit population increased from approximately 10,000 to more than 100,000 individuals. This increase was probably caused by a combination of fewer predatory cats, a rebounding abundance of plants, and a decline in the amount of virus distributed each year. By 2006, there were 130,000 rabbits and these herbivores had consumed so much vegetation that parts of the island resembled a closely cropped lawn.
These changes on the island underscored the importance of understanding all the interactions that introduced species can have on a community, including interactions among the introduced species themselves. In the case of Macquarie Island, it has become clear to scientists that removing the introduced cats was not enough to return the island community to its former condition. The introduced rabbits had to be removed as well. As a result, the Australian government agreed to spend $24 million to remove the rabbits from the island as well as several other introduced mammals, including mice and rats. Rabbit poison, distributed around the island in 2011, was very successful in killing the vast majority of the rabbits. In 2012 hunters and rabbit–hunting dogs were brought to the island to eliminate the few remaining rabbits. The success of this latest effort will determine whether an island that has been invaded by introduced predators and herbivores can ever return to its original state.
SOURCES: Bergstrom, D. M., et al. 2009. Indirect effects of invasive species removal devastate World Heritage Island. Journal of Applied Ecology 46: 83–81.
Dowding, J. E., et al. 2009. Cats, rabbits, Mxyoma virus, and vegetation on Macquarie Island: A comment on Bergstrom et al. (2009). Journal of Applied Ecology 46: 1129–1132.
Bergstrom, D. M., et al. 2009. Management implications of the Macquarie Island trophic cascade revisited: A reply to Dowding et al. (2009). Journal of Applied Ecology 46: 1133–1136.
http://www.abc.net.au/news/2012-04-19/rabbit-hunters-head-to-macquarie-island/3961270