15.3The Oxidation of Carbon Fuels Is an Important Source of Cellular Energy
The Oxidation of Carbon Fuels Is an Important Source of Cellular Energy
ATP serves as the principal immediate donor of free energy in biological systems rather than as a long-
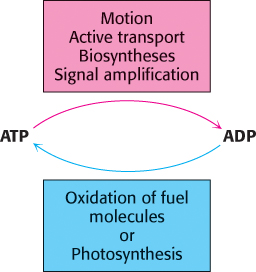
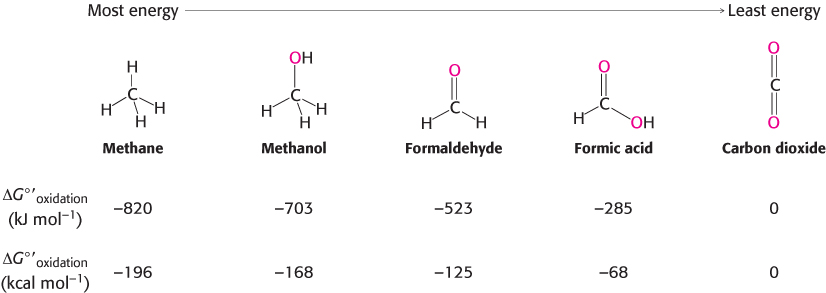
In aerobic organisms, the ultimate electron acceptor in the oxidation of carbon is O2 and the oxidation product is CO2. Consequently, the more reduced a carbon is to begin with, the more free energy is released by its oxidation. Figure 15.9 shows the ΔG°′ of oxidation for one-
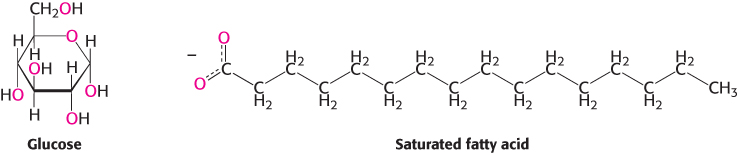
Fuel molecules are more complex (Figure 15.10) than the single-
Compounds with high phosphoryl-transfer potential can couple carbon oxidation to ATP synthesis
How is the energy released from the oxidation of a carbon compound converted into ATP? As an example, consider glyceraldehyde 3-
433
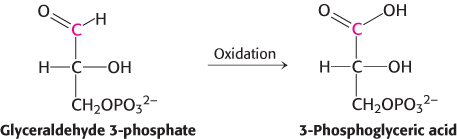
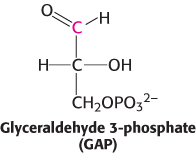
However, the oxidation does not take place directly. Instead, the carbon oxidation generates an acyl phosphate, 1,3-
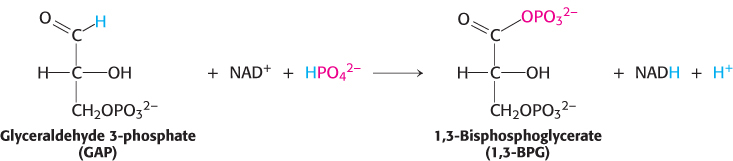
For reasons similar to those discussed for ATP, 1,3-
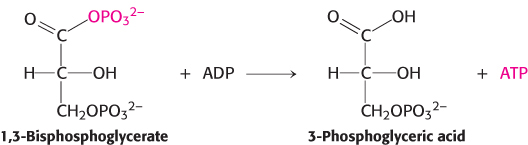
The energy of oxidation is initially trapped as a high-
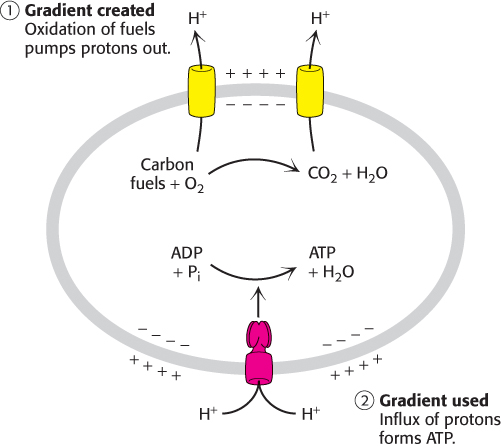
Ion gradients across membranes provide an important form of cellular energy that can be coupled to ATP synthesis
As described in Chapter 13, electrochemical potential is an effective means of storing free energy. Indeed, the electrochemical potential of ion gradients across membranes, produced by the oxidation of fuel molecules or by photosynthesis, ultimately powers the synthesis of most of the ATP in cells. In general, ion gradients are versatile means of coupling thermodynamically unfavorable reactions to favorable ones. Indeed, in animals, proton gradients generated by the oxidation of carbon fuels account for more than 90% of ATP generation (Figure 15.11). This process is called oxidative phosphorylation (Chapter 18). ATP hydrolysis can then be used to form ion gradients of different types and functions. The electrochemical potential of a Na+ gradient, for example, can be tapped to pump Ca2+ out of cells or to transport nutrients such as sugars and amino acids into cells.
434
Phosphates play a prominent role in biochemical processes
We have seen in Section 10.3, Chapter 14, and in this chapter the prominence of phosphoryl group transfer from ATP to acceptor molecules. How is it that phosphate came to play such a prominent role in biology? Phosphate and its esters have several characteristics that render it useful for biochemical systems. First, phosphate esters have the important property of being thermodynamically unstable while being kinetically stable. Phosphate esters are thus molecules whose energy release can be manipulated by enzymes. The stability of phosphate esters is due to the negative charges that make them resistant to hydrolysis in the absence of enzymes. This accounts for the presence of phosphate in the backbone of DNA. Furthermore, because phosphate esters are so kinetically stable, they make ideal regulatory molecules, added to proteins by kinases and removed only by phosphatases. Phosphates are also frequently added to metabolites that might otherwise diffuse through the cell membrane. Furthermore, even when transporters exist for unphosphorylated forms of a metabolite, the addition of a phosphate changes the geometry and polarity of the molecules so that they no longer fit in the binding sites of the transporters.
No other ions have the chemical characteristics of phosphate. Citrate is not sufficiently charged to prevent hydrolysis. Arsenate forms esters that are unstable and susceptible to spontaneous hydrolysis. Indeed, arsenate is poisonous to cells because it can replace phosphate in reactions required for ATP synthesis, generating unstable compounds and preventing ATP synthesis. Silicate is more abundant than phosphate, but silicate salts are virtually insoluble, and in fact, are used for biomineralization. Only phosphate has the chemical properties to meet the needs of living systems.
Energy from foodstuffs is extracted in three stages
Let us take an overall view of the processes of energy conversion in higher organisms before considering them in detail in subsequent chapters. Hans Krebs described three stages in the generation of energy from the oxidation of foodstuffs (Figure 15.12).
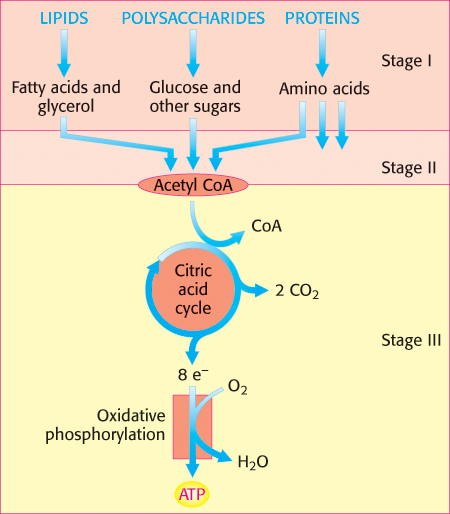
In the first stage, large molecules in food are broken down into smaller units in the process of digestion. Proteins are hydrolyzed to their 20 different amino acids, polysaccharides are hydrolyzed to simple sugars such as glucose, and lipids are hydrolyzed to glycerol and fatty acids. The degradation products are then absorbed by the cells of the intestine and distributed throughout the body. This stage is strictly a preparation stage; no useful energy is captured in this phase.
In the second stage, these numerous small molecules are degraded to a few simple units that play a central role in metabolism. In fact, most of them—
In the third stage, ATP is produced from the complete oxidation of the acetyl unit of acetyl CoA. The third stage consists of the citric acid cycle and oxidative phosphorylation, which are the final common pathways in the oxidation of fuel molecules. Acetyl CoA brings acetyl units into the citric acid cycle [also called the tricarboxylic acid (TCA) cycle or Krebs cycle], where they are completely oxidized to CO2. Four pairs of electrons are transferred (three to NAD+ and one to FAD) for each acetyl group that is oxidized. Then, a proton gradient is generated as electrons flow from the reduced forms of these carriers to O2, and this gradient is used to synthesize ATP (Chapters 17 and 18).
435