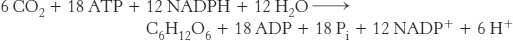20.1
The Calvin Cycle Synthesizes Hexoses from Carbon Dioxide and Water
As stated in Chapter 16, glucose can be formed from noncarbohydrate precursors, such as lactate and amino acids, by gluconeogenesis. The energy powering gluconeogenesis ultimately comes from previous catabolism of carbon fuels. In contrast, photosynthetic organisms can use the Calvin cycle to synthesize glucose from carbon dioxide gas and water, by using sunlight as an energy source. The Calvin cycle introduces into life all the carbon atoms that will be used as fuel and as the carbon backbones of biomolecules. Photosynthetic organisms are called autotrophs (literally, self-feeders) because they can convert sunlight into chemical energy, which they subsequently use to power their biosynthetic processes. Organisms that obtain energy from chemical fuels only are called heterotrophs, and such organisms ultimately depend on autotrophs for their fuel.
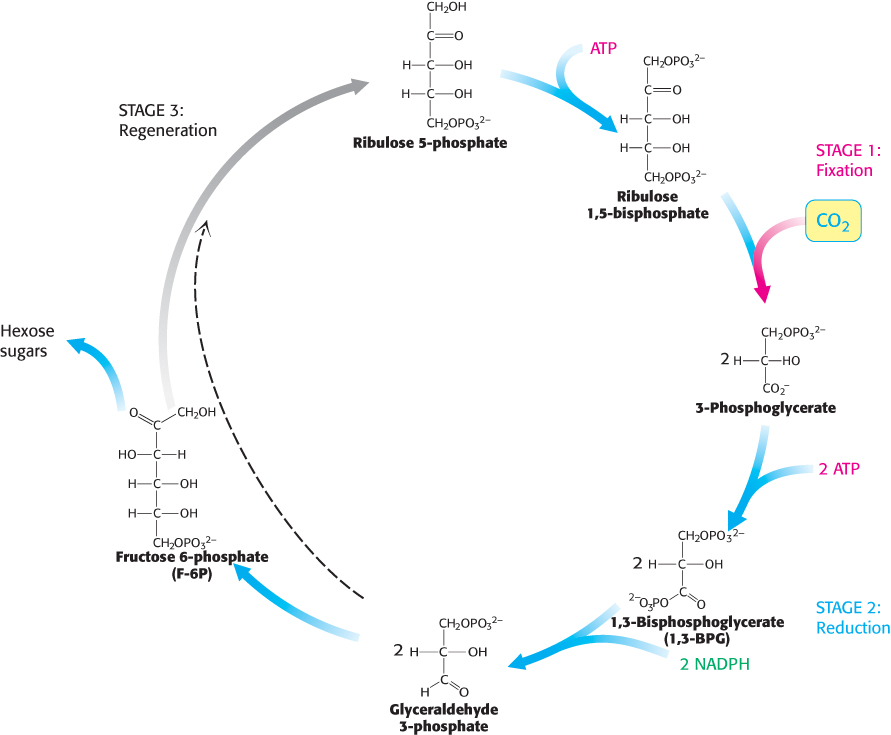
FIGURE 20.1Calvin cycle. The Calvin cycle consists of three stages. Stage 1 is the fixation of carbon by the carboxylation of ribulose 1,5-bisphosphate. Stage 2 is the reduction of the fixed carbon to begin the synthesis of hexose. Stage 3 is the regeneration of the starting compound ribulose 1,5-bisphosphate.
The fixation of CO2 by ribulose 1,5-bisphosphate to form two molecules of 3-phosphoglycerate;
The reduction of 3-phosphoglycerate to form hexose sugars; and
The regeneration of ribulose 1,5-bisphosphate so that more CO2 can be fixed.
This set of reactions takes place in the stroma of chloroplasts, the photosynthetic organelles.
Carbon dioxide reacts with ribulose 1,5-bisphosphate to form two molecules of 3-phosphoglycerate
The first step in the Calvin cycle is the fixation of CO2. This begins with the conversion of ribulose 1,5-bisphosphate into a highly reactive enediolate intermediate. The CO2 molecule condenses with the enediol intermediate to form an unstable six-carbon compound, which is rapidly hydrolyzed to two molecules of 3-phosphoglycerate.
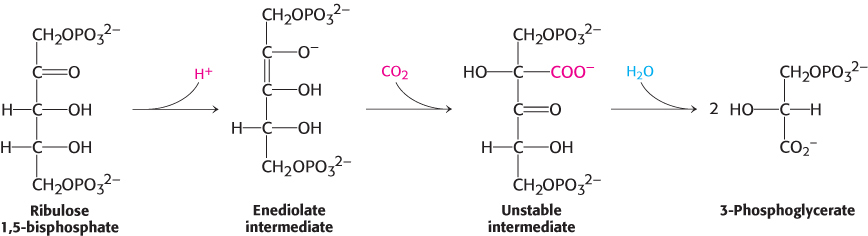
This highly exergonic reaction [ΔG°′ = −51.9 kJ mol−1 (−12.4 kcal mol−1)] is catalyzed by ribulose 1,5-bisphosphate carboxylase/oxygenase (usually called rubisco), an enzyme located on the stromal surface of the thylakoid membranes of chloroplasts. This important reaction is the rate-limiting step in hexose synthesis. In plants and green algae, rubisco consists of eight large (L, 55-kDa) subunits and eight small (S, 15-kDa) ones (Figure 20.2). Each L chain contains a catalytic site and a regulatory site. The S chains enhance the catalytic activity of the L chains. This enzyme is abundant in chloroplasts, accounting for approximately 30% of the total leaf protein in some plants. In fact, rubisco is the most abundant enzyme and probably the most abundant protein in the biosphere. Large amounts are present because rubisco is a slow enzyme; its maximal catalytic rate is only 3 s−1.
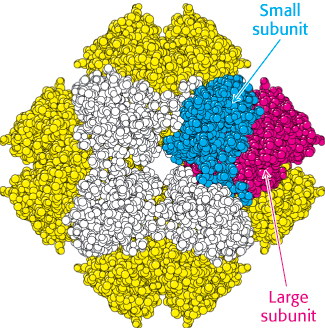
 FIGURE 20.2 Structure of rubisco. The enzyme ribulose 1,5-bisphosphate carboxylase/oxygenase (rubisco) comprises eight large subunits (one shown in red and the others in yellow) and eight small subunits (one shown in blue and the others in white). The active sites lie in the large subunits.
FIGURE 20.2 Structure of rubisco. The enzyme ribulose 1,5-bisphosphate carboxylase/oxygenase (rubisco) comprises eight large subunits (one shown in red and the others in yellow) and eight small subunits (one shown in blue and the others in white). The active sites lie in the large subunits.
[Drawn from 1RXO.pdb.]
Rubisco activity depends on magnesium and carbamate
Rubisco requires a bound divalent metal ion for activity, usually magnesium ion. Like the zinc ion in the active site of carbonic anhydrase (Section 9.2), this metal ion serves to activate a bound substrate molecule by stabilizing a negative charge. Interestingly, a CO2 molecule other than the substrate is required to complete the assembly of the Mg2+-binding site in rubisco. This CO2 molecule adds to the uncharged ε-amino group of lysine 201 to form a carbamate. This negatively charged adduct then binds the Mg2+ ion.
The metal center plays a key role in binding ribulose 1,5-bisphosphate and activating it so that it reacts with CO2 (Figure 20.3). Ribulose 1,5-bisphosphate binds to Mg2+ through its keto group and an adjacent hydroxyl group. This complex is readily deprotonated to form an enediolate intermediate. This reactive species, analogous to the zinc–hydroxide species in carbonic anhydrase, couples with CO2, forming the new carbon–carbon bond. The resulting 2-carboxy-3-keto-D-arabinitol 1,5-bisphosphate is coordinated to the Mg2+ ion through three groups, including the newly formed carboxylate. A molecule of H2O is then added to this β-ketoacid to form an intermediate that cleaves to form two molecules of 3-phosphoglycerate (Figure 20.4).
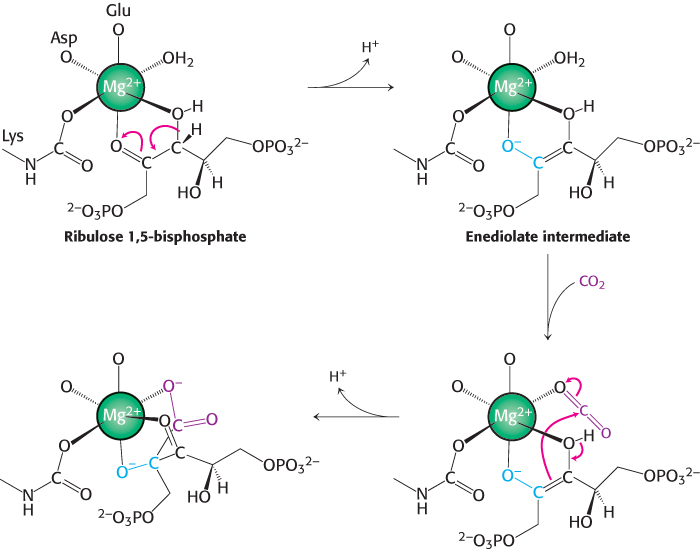
FIGURE 20.3Role of the magnesium ion in the rubisco mechanism. Ribulose 1,5-bisphosphate binds to a magnesium ion that is linked to rubisco through a glutamate residue, an aspartate residue, and the lysine carbamate. The coordinated ribulose 1,5-bisphosphate gives up a proton to form a reactive enediolate species that reacts with CO2 to form a new carbon–carbon bond.
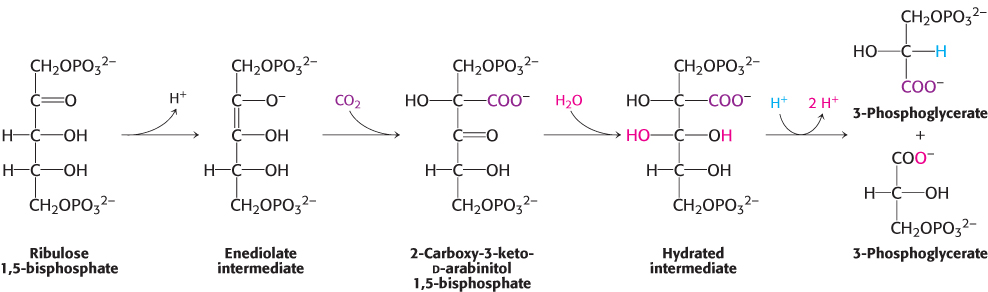
FIGURE 20.4Formation of 3-phosphoglycerate. The overall pathway for the conversion of ribulose 1,5 bisphosphate and CO2 into two molecules of 3-phosphoglycerate. Although the free species are shown, these steps take place on the magnesium ion.
Rubisco activase is essential for rubisco activity
In the absence of CO2, rubisco binds to its substrate, ribulose 1,5-bisphosphate, so tightly that its enzyme activity is inhibited. The enzyme rubisco activase uses ATP to induce structural changes in rubisco that allow release of the bound substrate and formation of the required carbamate. The ATP requirement of rubisco activase coordinates rubisco activity with the light reactions.
The activase contains a P-loop domain (Section 9.4) and assembles into a hexameric ring–like structure. The enzyme binds to the C-terminal end of the polypeptide chain that forms that large subunit. Using the energy of ATP hydrolysis, the C-terminal is transiently pulled in to the central pore of the activase. This releases the bound ribulose 1,5-bisphosphate and activates rubisco. Activase is a member of the large AAA family of ATPases that usually assemble into ring-shaped oligomeric structures and use the ATP hydrolysis to power conformational changes in their substrates. AAA ATPases play a variety of roles in biological systems. They are crucial for protein degradation (Chapter 23) and DNA replication (Chapter 28), and also function as molecular motors (Chapter 35).
Rubisco also catalyzes a wasteful oxygenase reaction: Catalytic imperfection
The reactive enediolate intermediate generated on the Mg2+ ion sometimes reacts with O2 instead of CO2; thus, rubisco also catalyzes a deleterious oxygenase reaction. The products of this reaction are phosphoglycolate and 3-phosphoglycerate (Figure 20.5). The rate of the carboxylase reaction is four times that of the oxygenase reaction under normal atmospheric conditions at 25°C; the stromal concentration of CO2 is then 10 μM and that of O2 is 250 μM. The oxygenase reaction, like the carboxylase reaction, requires that lysine 201 be in the carbamate form. Because this carbamate forms only in the presence of CO2, rubisco is prevented from catalyzing the oxygenase reaction exclusively when CO2 is absent.
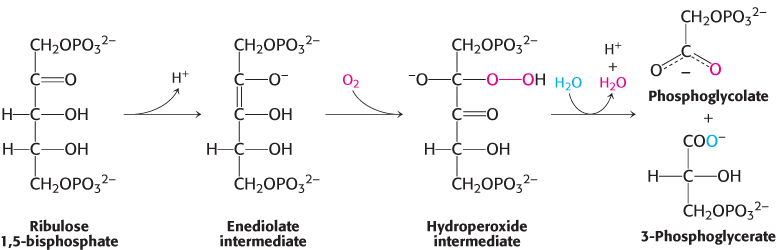
FIGURE 20.5A wasteful side reaction. The reactive enediolate intermediate on rubisco also reacts with molecular oxygen to form a hydroperoxide intermediate, which then proceeds to form one molecule of 3-phosphoglycerate and one molecule of phosphoglycolate.
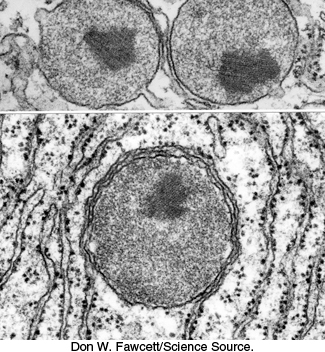
FIGURE 20.7Electron micrograph of three peroxisomes. The peroxisomes are surrounded by rough endoplasmic reticulum.
[Don W. Fawcett/Science Source.]
Phosphoglycolate is not a versatile metabolite. A salvage pathway recovers part of its carbon skeleton (Figure 20.6). A specific phosphatase converts phosphoglycolate into glycolate, which enters peroxisomes (also called microbodies; Figure 20.7). Glycolate is then oxidized to glyoxylate by glycolate oxidase, an enzyme with a flavin mononucleotide prosthetic group. The H2O2 produced in this reaction is cleaved by catalase to H2O and O2. Transamination of glyoxylate then yields glycine, which then enters the mitochondria. Two glycine molecules can unite to form serine with the release of CO2 and ammonium ion (NH4+). The ammonium ion, used in the synthesis of nitrogen-containing compounds, is salvaged by a glutamine synthetase reaction (Figure 20.6 and Section 23.3). Serine reenters the peroxisome,where it donates its ammonia to glyoxylate and is subsequently converted back into 3-phosphoglycerate.
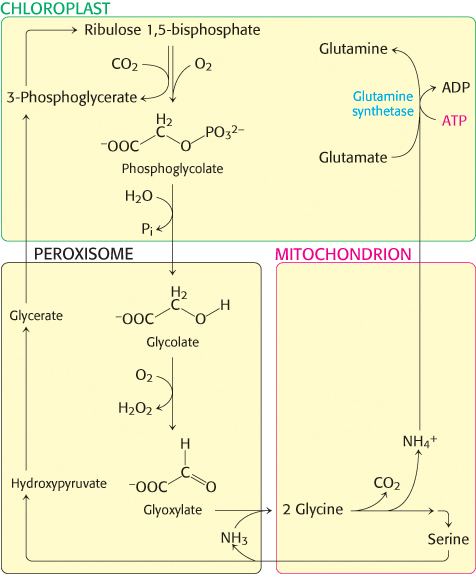
FIGURE 20.6Photorespiratory reactions. Phosphoglycolate is formed as a product of the oxygenase reaction in chloroplasts. After dephosphorylation, glycolate is transported into peroxisomes where it is converted into glyoxylate and then glycine. In mitochondria, two glycines are converted into serine, after losing a carbon as CO2 and ammonium ion. The serine is converted back into 3-phosphoglycerate and the ammonium ion is salvaged in chloroplasts.
This salvage pathway serves to recycle three of the four carbon atoms of two molecules of glycolate. However, one carbon atom is lost as CO2. This process is called photorespiration because O2 is consumed and CO2 is released. Photorespiration is wasteful because organic carbon is converted into CO2 without the production of ATP, NADPH, or another energy-rich metabolite. Although evolutionary processes have presumably enhanced the preference of rubisco for carboxylation—for instance, the rubisco of higher plants is eightfold as specific for carboxylation as that of photosynthetic bacteria—photorespiration still accounts for the loss of up to 25% of the carbon fixed.
Much research has been undertaken to generate recombinant forms of rubisco that display reduced oxygenase activity, but all such attempts have failed. This raises the following question: What is the biochemical basis of this inefficiency? Structural studies show that when the reactive enediolate intermediate is formed, loops close over the active site to protect the enediolate. A channel to the environment is maintained to allow access by CO2. However, like CO2, O2 is a linear molecule that also fits the channel. In essence, the problem lies not with the enzyme but in the unremarkable structure of CO2. CO2 lacks any chemical features that would allow discrimination between it and other gases such as O2, and thus the oxygenase activity is an inevitable failing of the enzyme. Another possibility exists, however. The oxygenase activity may not be an imperfection of the enzyme, but rather an imperfection in our understanding. Perhaps the oxygenase activity performs a biochemically important role that we have not yet discovered.
Hexose phosphates are made from phosphoglycerate, and ribulose 1,5-bisphosphate is regenerated
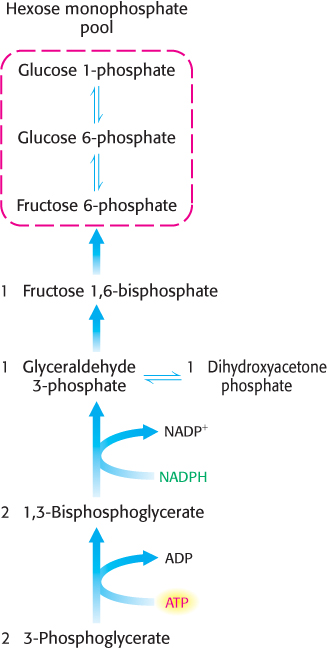
FIGURE 20.8Hexose phosphate formation. 3-Phosphoglycerate is converted into fructose 6-phosphate in a pathway parallel to that of gluconeogenesis.
The 3-phosphoglycerate product of rubisco is next converted into fructose 6-phosphate, which readily isomerizes to glucose 1-phosphate and glucose 6-phosphate. The mixture of the three phosphorylated hexoses is called the hexose monophosphate pool. The steps in this conversion (Figure 20.8) are like those of the gluconeogenic pathway (Figure 16.24), except that glyceraldehyde 3-phosphate dehydrogenase in chloroplasts, which generates glyceraldehyde 3-phosphate (GAP), is specific for NADPH rather than NADH. These reactions and that catalyzed by rubisco bring CO2 to the level of a hexose, converting CO2 into a chemical fuel at the expense of NADPH and ATP generated from the light reactions.
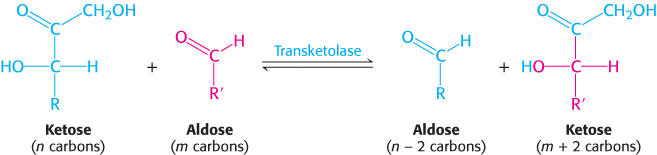
The third phase of the Calvin cycle is the regeneration of ribulose 1,5-bisphosphate, the acceptor of CO2 in the first step. The problem is to construct a five-carbon sugar from six-carbon and three-carbon sugars. A transketolase and an aldolase play the major role in the rearrangement of the carbon atoms. The transketolase, which we will see again in the pentose phosphate pathway, requires the coenzyme thiamine pyrophosphate (TPP) to transfer a two-carbon unit (CO CH2OH) from a ketose to an aldose.
CH2OH) from a ketose to an aldose.
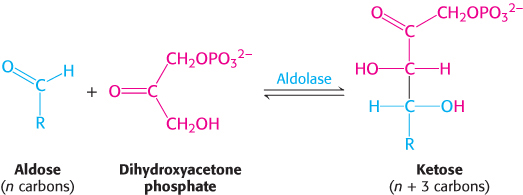
Aldolase, which we have already encountered in glycolysis (Section 16.1), catalyzes an aldol condensation between dihydroxyacetone phosphate (DHAP) and an aldehyde. This enzyme is highly specific for dihydroxyacetone phosphate, but it accepts a wide variety of aldehydes.
With these enzymes, the construction of the five-carbon sugar proceeds as shown in Figure 20.9.

FIGURE 20.9Formation of five-carbon sugars. First, transketolase converts a six-carbon sugar and a three-carbon sugar into a four-carbon sugar and a five-carbon sugar. Then, aldolase combines the four-carbon product and a three-carbon sugar to form a seven-carbon sugar. Finally, this seven-carbon sugar reacts with another three-carbon sugar to form two additional five-carbon sugars.
Finally, ribose 5-phosphate is converted into ribulose 5-phosphate by phosphopentose isomerase, whereas xylulose 5-phosphate is converted into ribulose 5-phosphate by phosphopentose epimerase. Ribulose 5-phosphate is converted into ribulose 1,5-bisphosphate through the action of phosphoribulokinase (Figure 20.10). The sum of these reactions is
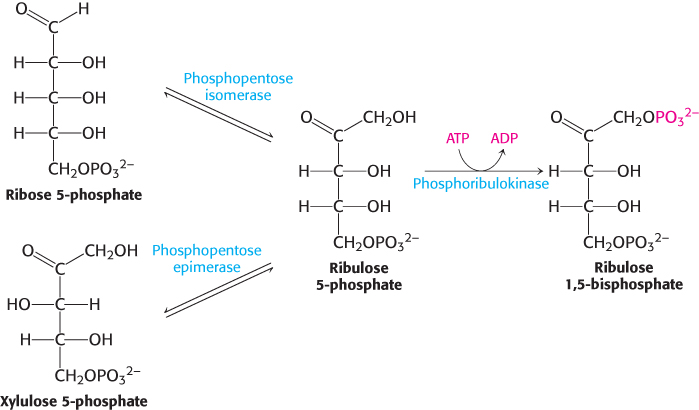
FIGURE 20.10Regeneration of ribulose 1,5-bisphosphate. Both ribose 5-phosphate and xylulose 5-phosphate are converted into ribulose 5-phosphate, which is then phosphorylated to complete the regeneration of ribulose 1,5-bisphosphate.
Figure 20.11 presents the required reactions with the proper stoichiometry to convert three molecules of CO2 into one molecule of DHAP. However, two molecules of DHAP are required for the synthesis of a member of the hexose monophosphate pool. Consequently, the cycle as presented must take place twice to yield a hexose monophosphate. The outcome of the Calvin cycle is the generation of a hexose and the regeneration of the starting compound, ribulose 1,5-bisphosphate. In essence, ribulose 1,5-bisphosphate acts catalytically, similarly to oxaloacetate in the citric acid cycle.
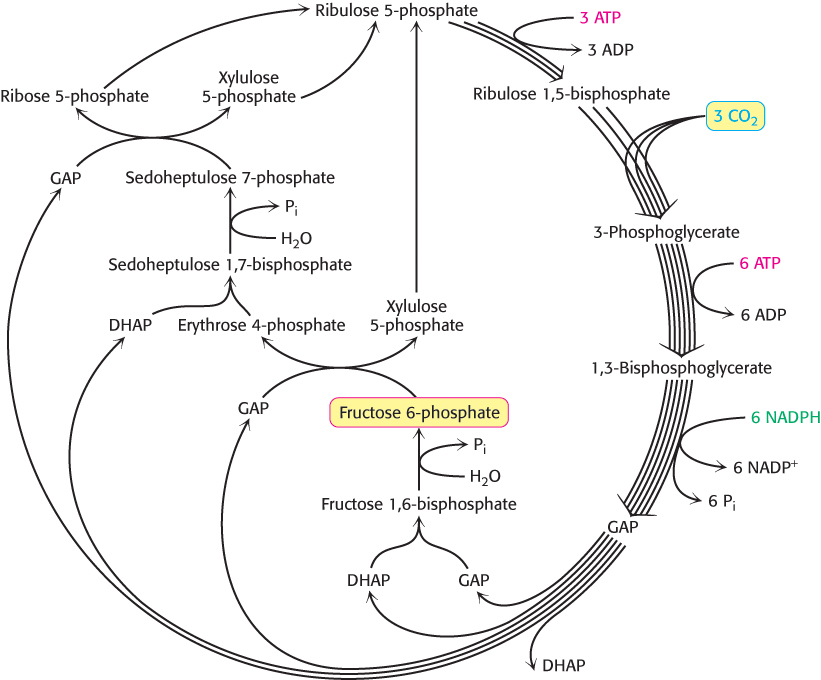
FIGURE 20.11Calvin cycle. The diagram shows the reactions necessary with the correct stoichiometry to convert three molecules of CO2 into one molecule of dihydroxyacetone phosphate (DHAP). The cycle is not as simple as presented in Figure 20.1; rather, it entails many reactions that lead ultimately to the synthesis of glucose and the regeneration of ribulose 1,5-bisphosphate.
[Information from J. R. Bowyer and R. C. Leegood. “Photosynthesis,” in Plant Biochemistry, P. M. Dey and J. B. Harborne, Eds. (Academic Press, 1997), p. 85.]
Three ATP and two NADPH molecules are used to bring carbon dioxide to the level of a hexose
What is the energy expenditure for synthesizing a hexose? Six rounds of the Calvin cycle are required, because one carbon atom is reduced in each round. Twelve molecules of ATP are expended in phosphorylating 12 molecules of 3-phosphoglycerate to 1,3-bisphosphoglycerate, and 12 molecules of NADPH are consumed in reducing 12 molecules of 1,3-bisphosphoglycerate to glyceraldehyde 3-phosphate. An additional six molecules of ATP are spent in regenerating ribulose 1,5-bisphosphate.
We can now write a balanced equation for the net reaction of the Calvin cycle:
Thus, three molecules of ATP and two molecules of NADPH are consumed in incorporating a single CO2 molecule into a hexose such as glucose or fructose.
Starch and sucrose are the major carbohydrate stores in plants
What are the fates of the members of the hexose monophosphate pool? These molecules are used in a variety of ways, but there are two primary roles. Plants contain two major storage forms of sugar: starch and sucrose. Starch, like its animal counterpart glycogen, is a polymer of glucose residues, but it is less branched than glycogen because it contains a smaller proportion of α-1,6-glycosidic linkages (Section 11.2). Another difference is that ADP-glucose, not UDP-glucose, is the activated precursor. Starch is synthesized and stored in chloroplasts.
In contrast, sucrose (common table sugar), a disaccharide, is synthesized in the cytoplasm. Plants lack the ability to transport hexose phosphates across the chloroplast membrane, but they are able to transport triose phosphates from chloroplasts to the cytoplasm. Triose phosphate intermediates such as glyceraldehyde 3-phosphate cross into the cytoplasm in exchange for phosphate through the action of an abundant triose phosphate-phosphate antiporter. Fructose 6-phosphate formed from triose phosphates joins the glucose unit of UDP-glucose to form sucrose 6-phosphate (Figure 20.12). The hydrolysis of the phosphate ester by sucrose phosphatase yields sucrose, a readily transportable and mobilizable sugar that is stored in many plant cells, such as those in sugar beets and sugar cane.
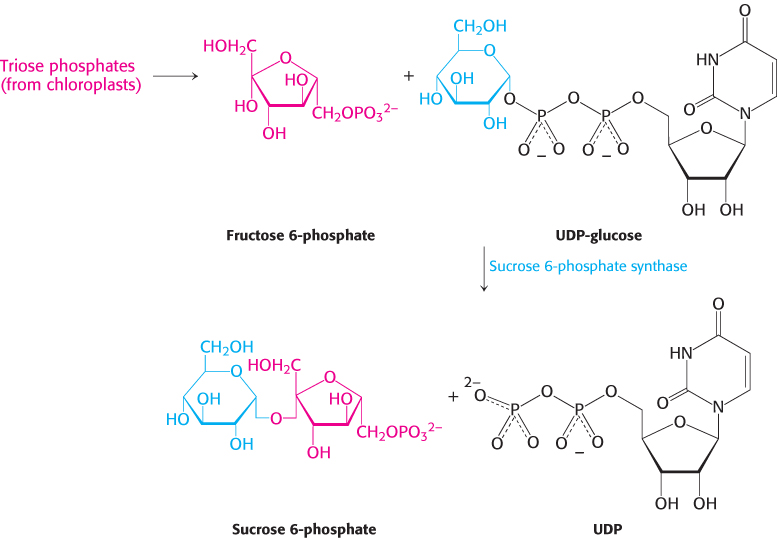
FIGURE 20.12Synthesis of sucrose. Sucrose 6-phosphate is formed by the reaction between fructose 6-phosphate and the activated intermediate uridine diphosphate glucose (UDP-glucose). Sucrose phosphatase subsequently generates free sucrose (not shown).



 FIGURE 20.2 Structure of rubisco. The enzyme ribulose 1,5-
FIGURE 20.2 Structure of rubisco. The enzyme ribulose 1,5-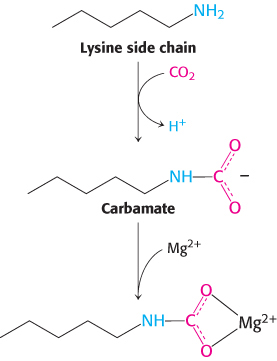






 CH2OH) from a ketose to an aldose.
CH2OH) from a ketose to an aldose.